
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Chapter 9.Phase Diagram Ill Review: 是 Immiscibility:不溶性 Spinodal Points:拐点 Peritectic Phase diagram:包晶相图 Compounds:化合物 AIJIAO TONG UNIVE A Peng Zhang NoV25,2016
Chapter 9. Phase Diagram III Review: Immiscibility: 不溶性 Spinodal Points:拐点 Peritectic Phase diagram:包晶相图 Compounds:化合物 1 Peng Zhang Nov 25, 2016
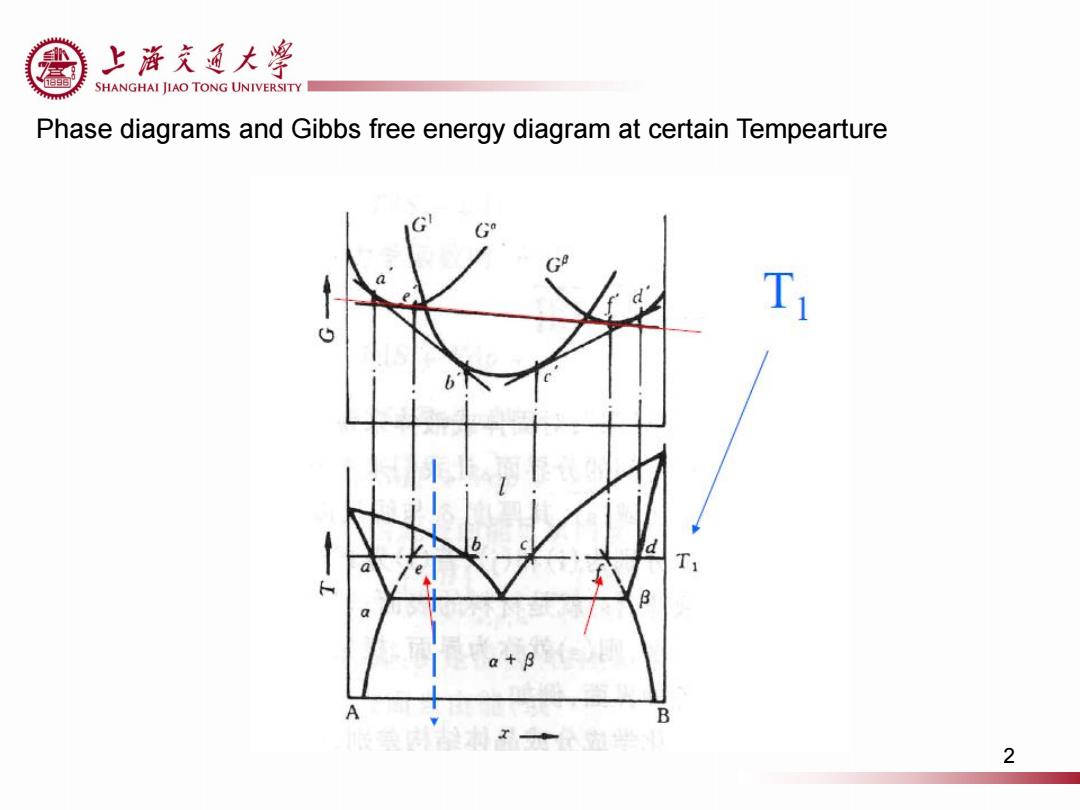
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phase diagrams and Gibbs free energy diagram at certain Tempearture G G T a+8 A B 2
Phase diagrams and Gibbs free energy diagram at certain Tempearture 2
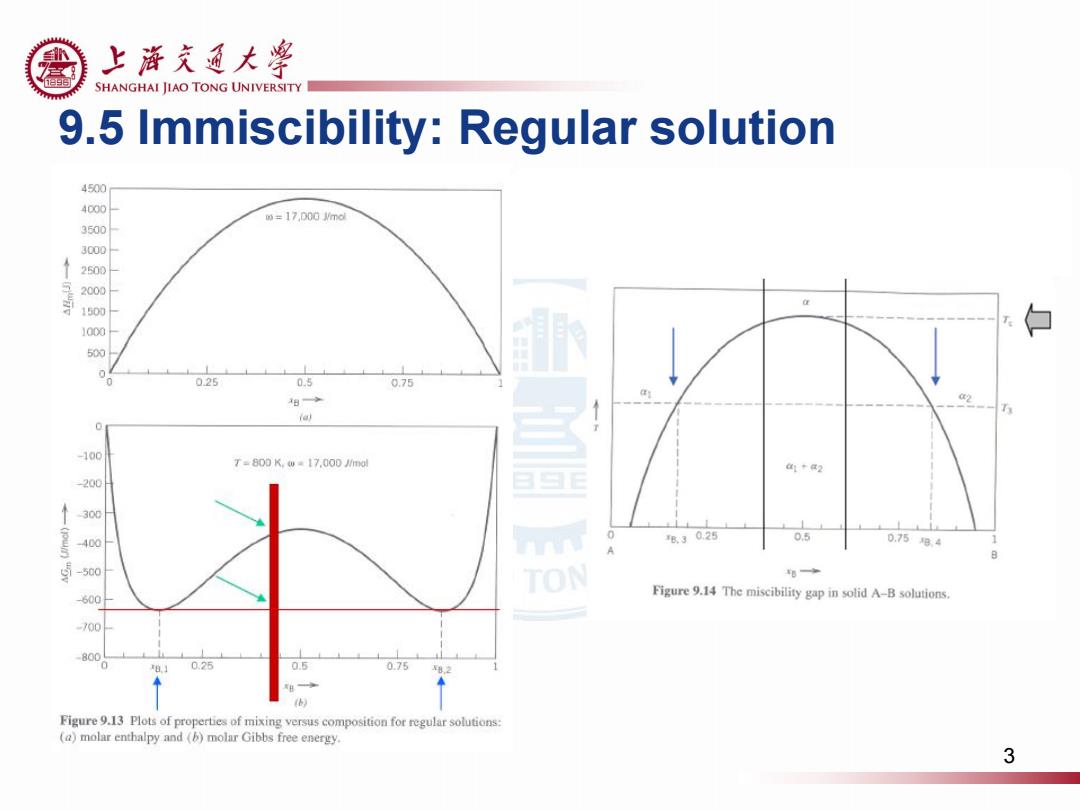
上游克通大学 SHANGHAI JIAO TONG UNIVERSITY 9.5 Immiscibility:Regular solution 4500 4000 m=17,D00md 3500 3000 2500 2000 1500 1000 白 500 025 0.5 0.75 出 ia) 0 -100 T=800K.w=17.000/m0 1t2 200 300 400 ®3025 0.5 0.758.4 1 -500 TON 想◆ -600 Figure 9.14 The miscibility gap in solid A-B solutions. 700 800 0.25 0.75 Figure 9.13 Plots of properties of mixing versus composition for regular solutions: (a)molar enthalpy and (b)molar Gibbs free energy. 3
9.5 Immiscibility: Regular solution 3
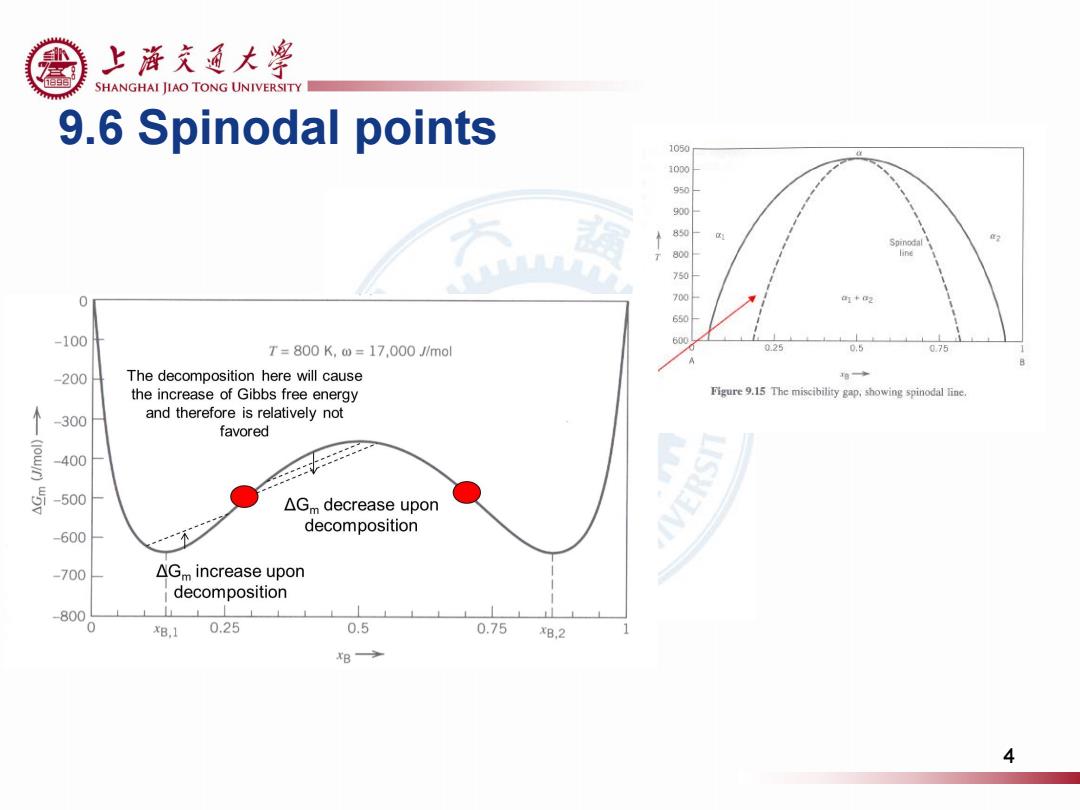
上游充通大粤 色 SHANGHAI JIAO TONG UNIVERSITY 9.6 Spinodal points 1050 1000 line 750 0 -100 600 T=800K,w=17,000 J/mol 0.25 0.5 0.75 -200 The decomposition here will cause the increase of Gibbs free energy Figure 9.15 The miscibility gap,showing spinodal line. 300 and therefore is relatively not favored 400 -500 △Gm decrease upon decomposition -600 -700 △Gm increase upon decomposition -800 0 xB.1 0.25 0.5 0.75 XB.2 xB→ 4
9.6 Spinodal points 4
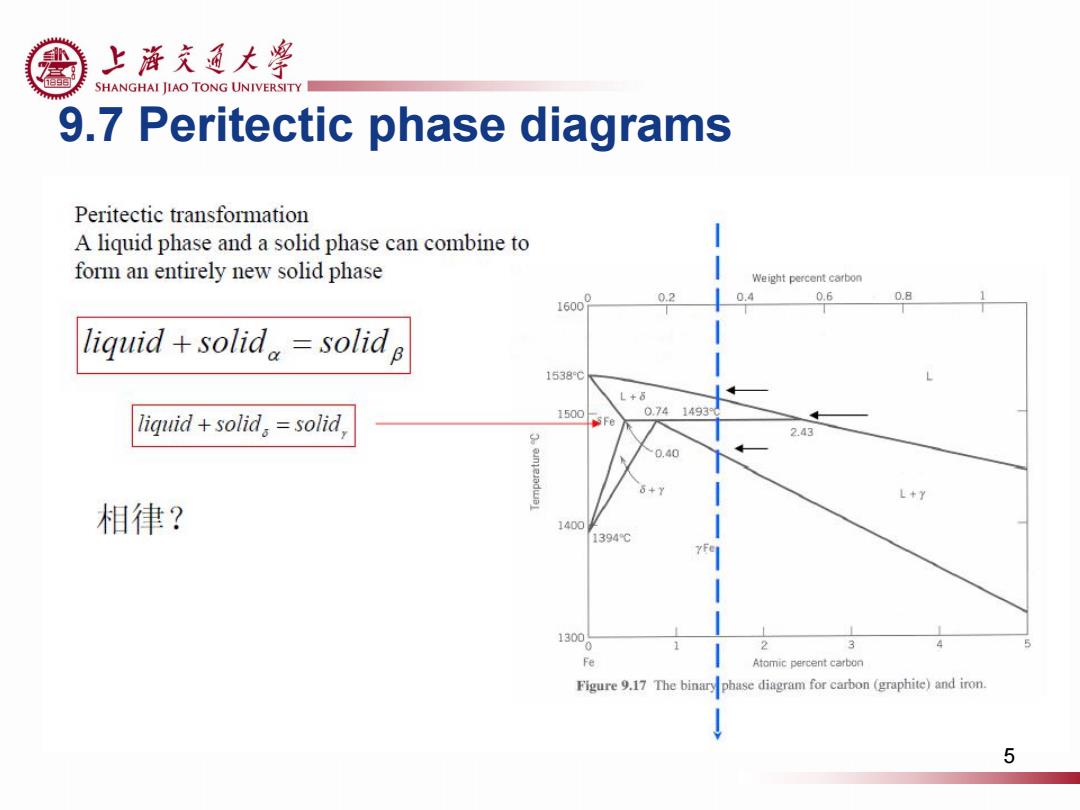
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY 9.7 Peritectic phase diagrams Peritectic transformation A liquid phase and a solid phase can combine to form an entirely new solid phase Weight percent carbon 02 0.4 0.6 0.8 liquid+solid。=solid B 1538℃ L+6 0.741493 1 iquid+solid。=solid 1500 5Fe 2.43 0.40 onjeradwBl 6+y L+7 相律? 1400 1394℃ YFe 1300 2 Fe Atomic percent carbon Figure 9.17 The binary phase diagram for carbon(graphite)and iron. 5
9.7 Peritectic phase diagrams 5