
Outline for Chapter 9 图 上帝支是人学 Chapter 9:Eutectoid and Reverse Eutectoid 9.1 Reverse Eutectoid Transformations 9.1.1 Intro to austenite in Fe-C system 9.1.2 Formation mechanism of austenite 9.1.3 Kinetics of austenitization 9.1.4 Austenite grain growth and its control 9.2 Eutectoid Transformations K.Chen 3
3

Glossary 图 上海哀通大学 Homogeneous/Homogeneity Spontaneous growth Inhomogeneous/Inhomogeneity Isothermal Heterogeneous/Heterogeneity √Lamella/Lamellae Eutectoid/Reverse eutectoid Lamellar/Interlamellar Ferrite/Cementite Migration Pearlite/Spheroidite Residual/Remaining Austenite/Austenitization Incubation time Normalizing √Proeutectoid phase Full annealing Hypereutectoid Interstice A0 √Hypoeutectoid Octahedral/Octahedron Tetrahedral/Tetrahedron Orthorhombic Concentration gradient K.Chen 4
4

Intro 上帝支通人学 JLAO TONG UNIN Typical processes for eutectoid transformation: 。Normalizing(正火) Heating at least 55C above the upper critical temperature-i.e., above A for compositions less than the eutectoid (0.76 wt%C).and above Accm for compositions greater than the eutectoid.After sufficient time has been allowed for the alloy to completely transform to austenite-the treatment is terminated by cooling in air Full Annealing(退: Heating to a temperature of about 50C above Ac3(to form austenite) for compositions less than the eutectoid,or,for compositions in excess of the eutectoid.50C above the A,line (to form austenite and Fe3C phases);then furnace cooled(for several hours) For increased machinability and formability In both processes,austenitization occurs before eutectoid reactions a +Fe3C-Y K.Chen 5
5
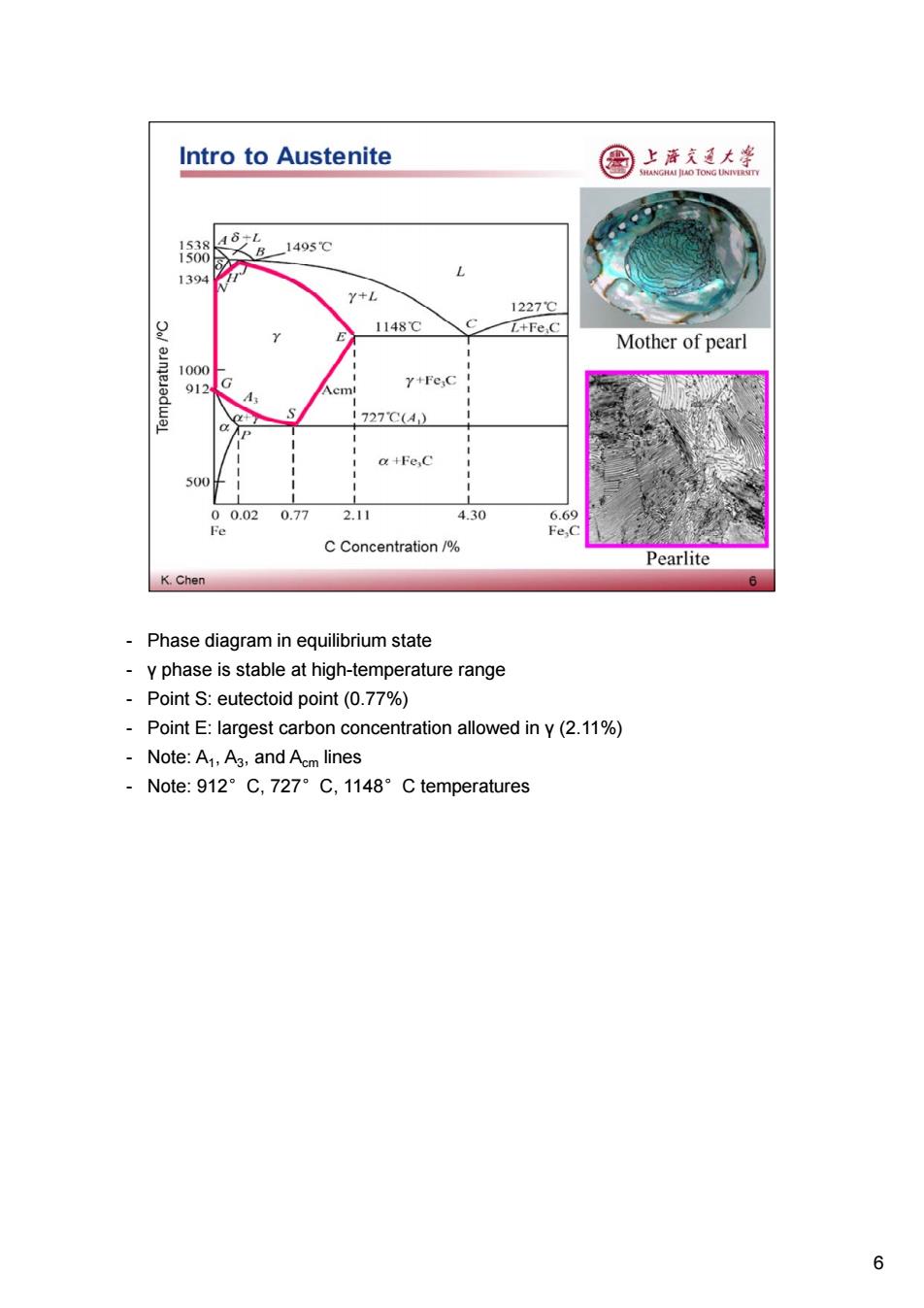
Intro to Austenite 图 上海充通大学 HAI JIAO TONG UNIVI 1538 AδL 1495℃ 1500 1394 Y+L 1227℃ 皇 1148℃ L+Fe C Mother of pearl 1000 912 Y+Fe C cm a+ 1727C(A) a +Fe,C 500 00.020.77 2.11 4.30 6.69 Fe Fe,C C Concentration/% Pearlite K.Chen 6 Phase diagram in equilibrium state y phase is stable at high-temperature range Point S:eutectoid point(0.77%) Point E:largest carbon concentration allowed in y(2.11%) Note:A1,A3,and Acm lines -Note:912°C,727°C,1148°C temperatures 6
- Phase diagram in equilibrium state - γ phase is stable at high-temperature range - Point S: eutectoid point (0.77%) - Point E: largest carbon concentration allowed in γ (2.11%) - Note: A1, A3, and Acm lines - Note: 912°C, 727°C, 1148°C temperatures 6

Intro to Austenite 图 上游充通大学 AI JLAO TONG UNI Actual transformation temperatures: Heating:Aci,Ac3.Accm a+yG Cooling:Ar,Ar3,Arcm Y+FeC Ac stands for arret chauffant(stop heating) A,stands for arret refroidissement(stop +Fe.C cooling) Q weX 100 K.Chen In practice,there is a temperature delay. Degree of delay depends on the heating/cooling rate.Higher rate results in higher degree of delay. 7
- In practice, there is a temperature delay. - Degree of delay depends on the heating/cooling rate. Higher rate results in higher degree of delay. 7