
Contents of Today S.J.T.0. Phase Transformation and Applications Continue /Review previous 2nd law Entropy Reversible process Carnot cycle etc. SJTU Thermodynamics of Materials Spring2o08©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I Contents of Today Continue / Review previous 2nd law Entropy Reversible process Carnot cycle etc
1.18 Enthalpies of Formation(1) S.J.T.0. Phase Transformation and Applications The enthalpy change of a material between states 1 and 2: △H=H2-H1 We could consider the mass itself to be a form of energy. Standard state A reference state:to assign a value of zero to enthalpy for certain materials in that reference state. Elements in their equilibrium states at 298 K and one atmosphere pressure. Ex., Enthalpy of diatomic oxygen at 298 K and one atm:ZERO Enthalpy of monatomic oxygen at 298 K and one atm:NOT ZERO Enthalpy of graphite at 298 K and one atm:ZERO Enthalpy of diamond at 298 K and one atm:NOT ZERO SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I 1.18 Enthalpies of Formation (1) The enthalpy change of a material between states 1 and 2: H H2 H1 We could consider the mass itself to be a form of energy. A reference state: to assign a value of zero to enthalpy for certain materials in that reference state. Elements in their equilibrium states at 298 K and one atmosphere pressure. Ex., Enthalpy of diatomic oxygen at 298 K and one atm: ZERO Enthalpy of monatomic oxygen at 298 K and one atm: NOT ZERO Enthalpy of graphite at 298 K and one atm: ZERO Enthalpy of diamond at 298 K and one atm: NOT ZERO Standard state
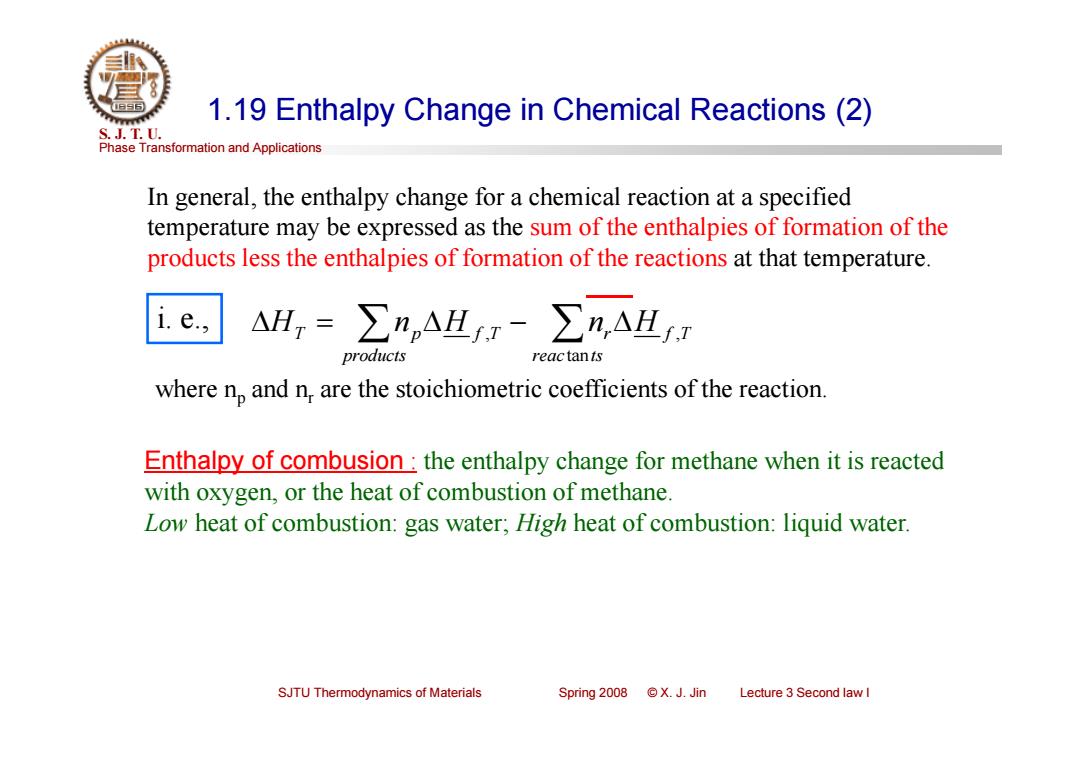
1.19 Enthalpy Change in Chemical Reactions(2) S.J.T.0. Phase Transformation and Applications In general,the enthalpy change for a chemical reaction at a specified temperature may be expressed as the sum of the enthalpies of formation of the products less the enthalpies of formation of the reactions at that temperature. △H=∑n,AHI-∑n,AH.T products reactants where np and n,are the stoichiometric coefficients of the reaction. Enthalpy of combusion the enthalpy change for methane when it is reacted with oxygen,or the heat of combustion of methane. Low heat of combustion:gas water;High heat of combustion:liquid water. SJTU Thermodynamics of Materials Spring 2008 ©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I 1.19 Enthalpy Change in Chemical Reactions (2) reac ts r f T products HT np H f T n H tan , , i. e., In general, the enthalpy change for a chemical reaction at a specified temperature may be expressed as the sum of the enthalpies of formation of the products less the enthalpies of formation of the reactions at that temperature. where np and nr are the stoichiometric coefficients of the reaction. Enthalpy of combusion : the enthalpy change for methane when it is reacted with oxygen, or the heat of combustion of methane. Low heat of combustion: gas water; High heat of combustion: liquid water
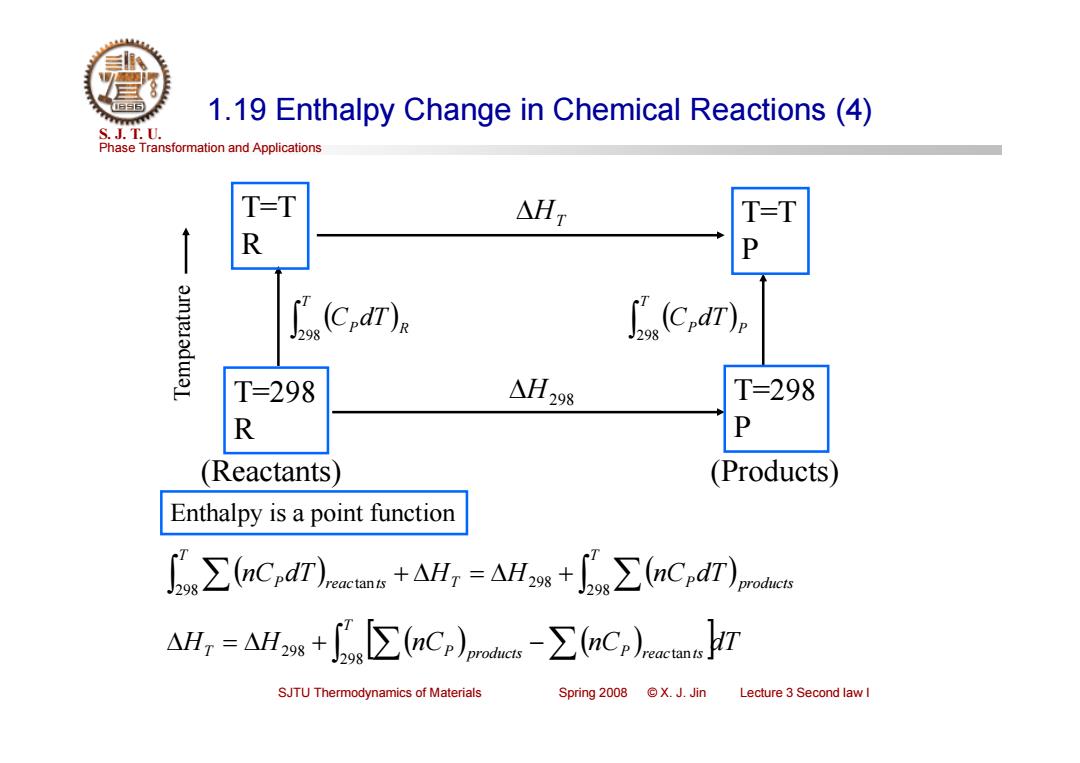
1.19 Enthalpy Change in Chemical Reactions (4) S.J.T.0. Phase Transformation and Applications T-T △HT T-T R 3mjeladwaL .(C). 以c,ar) T=298 △H298 T=298 R P Reactants (Products) Enthalpy is a point function ∑(nCrdT))ncma+AH,=AHw+f∑6 nCndT)p AH,=AHs+g∑Cr-∑6nCr)rmrr SJTU Thermodynamics of Materials Spring 2008 ©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I 1.19 Enthalpy Change in Chemical Reactions (4) T=T R T=298 R T=T P T=298 P H298 HT T P R C dT 298 T P P C dT 298 Temperature (Reactants) (Products) Enthalpy is a point function T T P products T P reac ts nC dT H H nC dT 298 298 298 tan H H nC nC dT T T P products P reac ts 298 298 tan
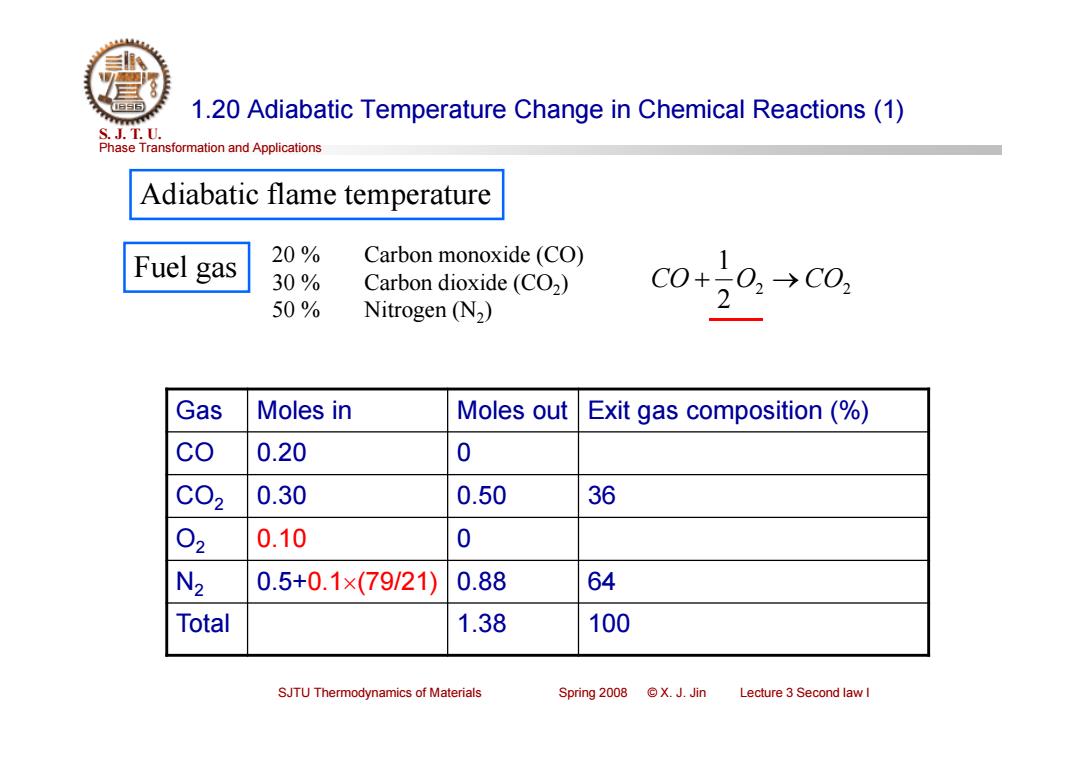
1.20 Adiabatic Temperature Change in Chemical Reactions(1) S.J.T.0. Phase Transformation and Applications Adiabatic flame temperature Fuel gas 20% Carbon monoxide (CO) 30% Carbon dioxide (CO2) 50% C0+20,→c0, Nitrogen (N2) 2 Gas Moles in Moles out Exit gas composition (% co 0.20 0 C02 0.30 0.50 36 02 0.10 0 N2 0.5+0.1×(79/21) 0.88 64 Total 1.38 100 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I 1.20 Adiabatic Temperature Change in Chemical Reactions (1) Adiabatic flame temperature Gas Moles in Moles out Exit gas composition (%) CO 0.20 0 CO2 0.30 0.50 36 O2 0.10 0 N2 0.5+0.1(79/21) 0.88 64 Total 1.38 100 Fuel gas 20 % Carbon monoxide (CO) 30 % Carbon dioxide (CO2) 50 % Nitrogen (N2) 2 2 2 1 CO O CO