
4.2 The application of the standard equilibrium constant 4.2.1 Determining the extent of a chemical reaction 4.2.2 Predicting the direction of a chemical reaction -4.2.3 Calculating equilibrium concentrations from K values
4.2.1 Determining the extent of a chemical reaction §4.2 The application of the standard equilibrium constant 4.2.3 Calculating equilibrium concentrations from K values 4.2.2 Predicting the direction of a chemical reaction
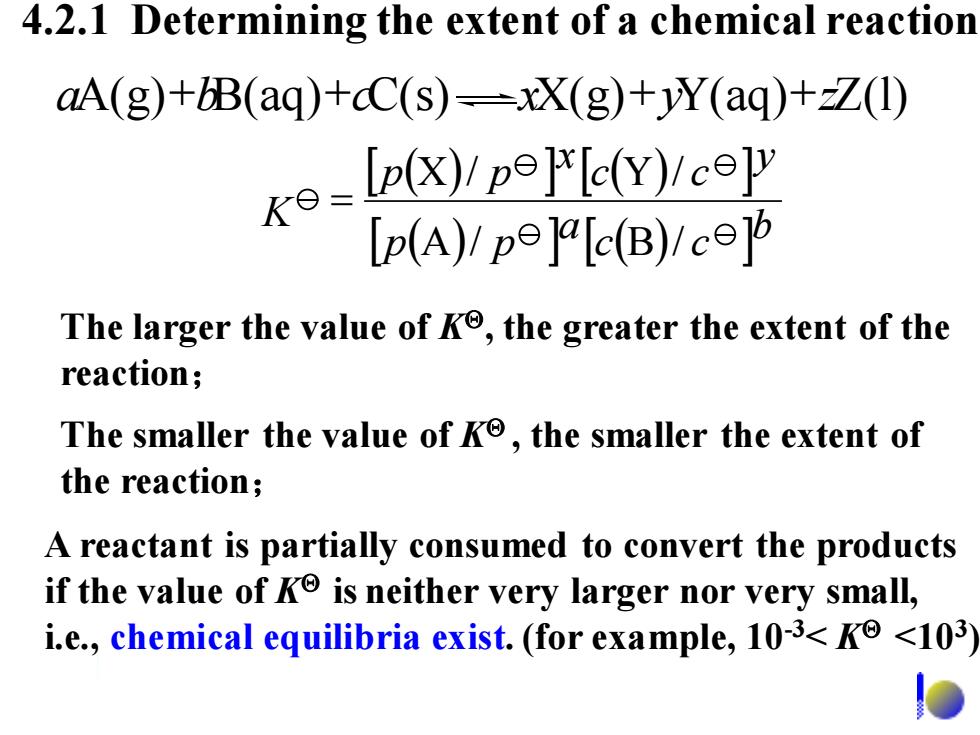
4.2.1 Determining the extent of a chemical reaction aA(g)+B(aq)+cC(s)-xX(g)+Y(aq)+zZ(1) -p)cop [p(A)/pejalc(B)!c] The larger the value of Ke,the greater the extent of the reaction; The smaller the value of Ko,the smaller the extent of the reaction; A reactant is partially consumed to convert the products if the value of K is neither very larger nor very small, i.e.,chemical equilibria exist.(for example,103<K<103)
4.2.1 Determining the extent of a chemical reaction The larger the value of K, the greater the extent of the reaction; A reactant is partially consumed to convert the products if the value of K is neither very larger nor very small, i.e., chemical equilibria exist. (for example, 10-3< K <103 ) The smaller the value of K , the smaller the extent of the reaction; K ( ) ( ) ( ) ( ) b c c a p p y c c x p p A / B / X / Y / = aA(g)+bB(aq)+cC(s) xX(g)+yY(aq)+zZ(l)

4.2.2 Predicting the direction of a chemical reaction Reaction quotient((反应商): For any chemical reaction: aA(g)+bB(aq)+cC(s)=xX(g)+yY(aq)+zZ(1) the reaction quotient J is J延[p,(X/p°]'[c,Y)/e]p [P,(A)/P][c,B)/ce] p=100kpa c=1 mol.I-1
For any chemical reaction: 4.2.2 Predicting the direction of a chemical reaction the reaction quotient J is aA (g)+ bB(aq)+cC(s) xX(g)+yY(aq)+zZ(l) b i a i y i x i p p c c p p c c J [ (A)/ ] [ (B)/ ] [ (X) / ] [ (Y)/ ] = def Reaction quotient(反应商): p = 100kpa c = 1 mol.l-1
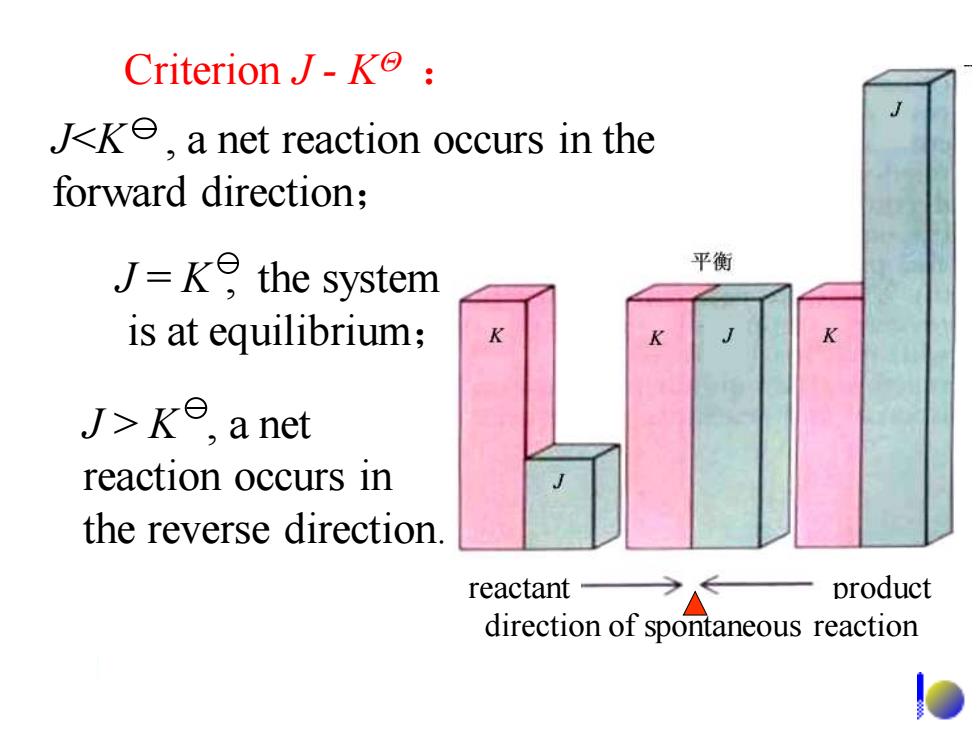
Criterion J-K: Jk,a net reaction occurs in the forward direction; J=ke the system 平衡 is at equilibrium; J>ke,a net reaction occurs in the reverse direction. reactant- product direction of spontaneous reaction
Criterion J - K : J = K , the system is at equilibrium; J > K , a net reaction occurs in the reverse direction. reactant product direction of spontaneous reaction J<K , a net reaction occurs in the forward direction;
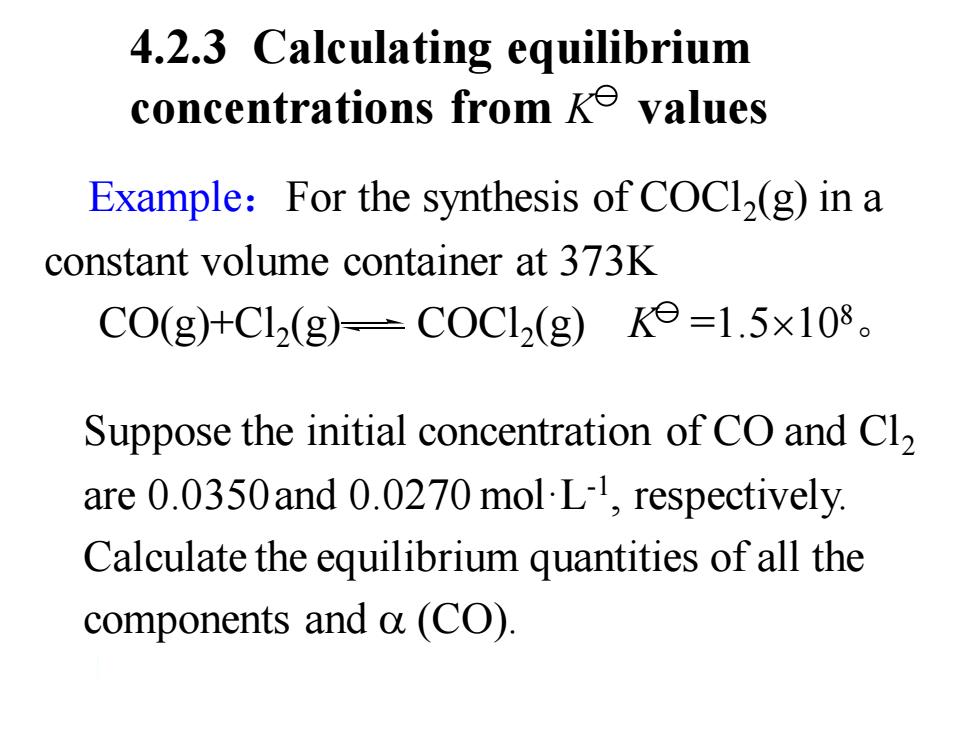
4.2.3 Calculating equilibrium concentrations from ke values Example:For the synthesis of COCl2(g)in a constant volume container at 373K C0(g)+Cl2(g)=C0Cl2(g)Ke=1.5×108。 Suppose the initial concentration of CO and Cl2 are 0.0350and 0.0270 mol L-,respectively. Calculate the equilibrium quantities of all the components and a (CO)
4.2.3 Calculating equilibrium concentrations from K values Suppose the initial concentration of CO and Cl2 are 0.0350and 0.0270 mol·L-1 , respectively. Calculate the equilibrium quantities of all the components and (CO). Example:For the synthesis of COCl2 (g) in a constant volume container at 373K CO(g)+Cl2 (g) COCl2 (g) K =1.5108