Chapter 8 Atomic Structure 8.1 The atomic structure of hydrogen x 8.2 The atomic structure of many-electron atoms x 8.3 Periodic law of elements
8.1 The atomic structure of hydrogen 8.2 The atomic structure of many-electron atoms 8.3 Periodic law of elements Chapter 8 Atomic Structure

8.1 The atomic structure of hydrogen -8.1.1 The hydrogen spectrum and Bohr's theory 8.1.2 The dual nature of the electron 8.1.3 Schrodinger equation and quantum numbers 8.1.4 The ground state of H atoms 8.1.5 The excited state of HAtoms 返 回
8.1.1 The hydrogen spectrum and Bohr’s theory 8.1 The atomic structure of hydrogen 8.1.5 The excited state of H Atoms 8.1.4 The ground state of H atoms 8.1.3 Schrödinger equation and quantum numbers 8.1.2 The dual nature of the electron
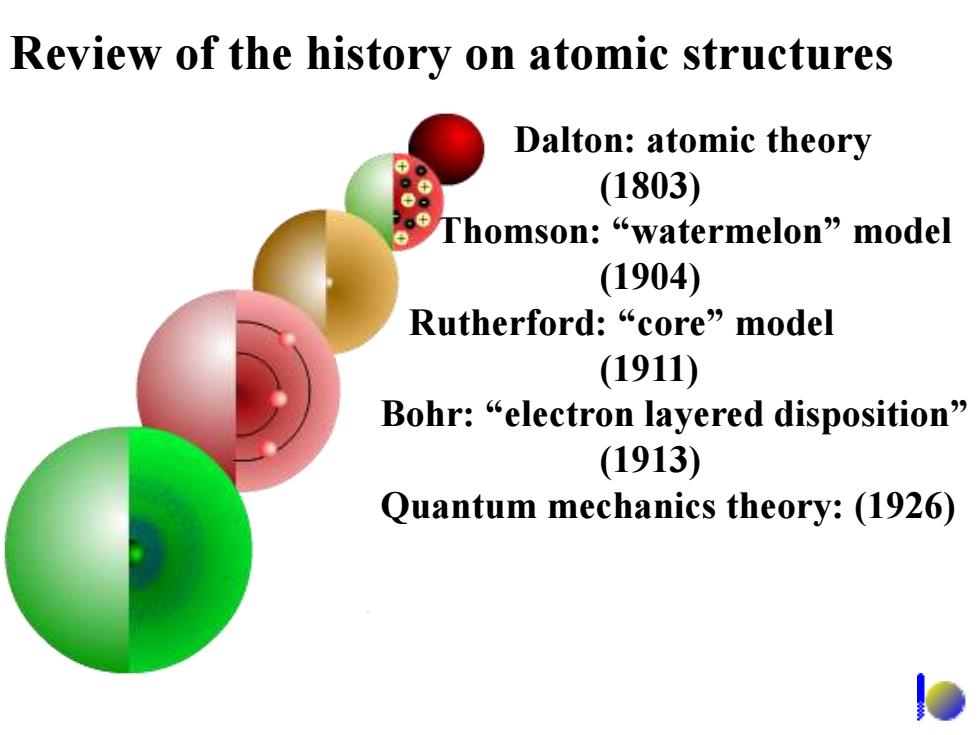
Review of the history on atomic structures Dalton:atomic theory (1803) 。 Thomson:“watermelon”nodel (1904) Rutherford:“core”nodel (1911) Bohr:“electron layered disposition” (1913) Quantum mechanics theory:(1926)
Review of the history on atomic structures Dalton: atomic theory (1803) Thomson: “watermelon” model (1904) Rutherford: “core” model (1911) Bohr: “electron layered disposition” (1913) Quantum mechanics theory: (1926)
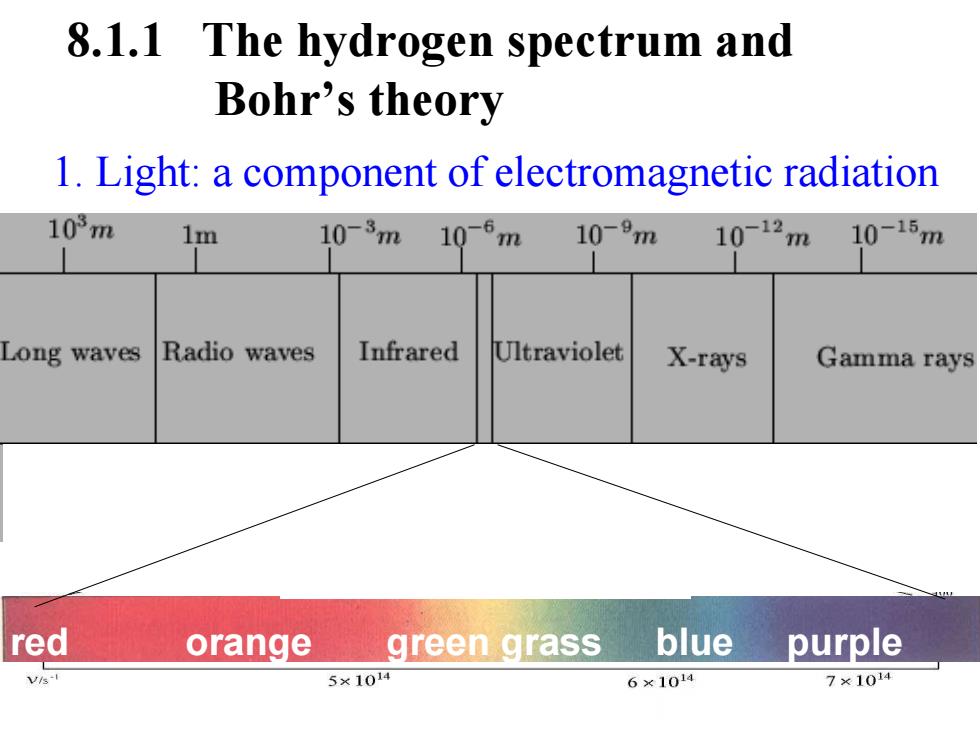
8.1.1 The hydrogen spectrum and Bohr's theory 1.Light:a component of electromagnetic radiation 103m 1m 10-3m10-6m 10-9m 10-12m 10-15m Long waves Radio waves Infrared Ultraviolet X-rays Gamma rays red orange green grass blue purple Vis-I 5×104 6×1014 7×104
1. Light: a component of electromagnetic radiation 8.1.1 The hydrogen spectrum and Bohr’s theory red orange green grass blue purple
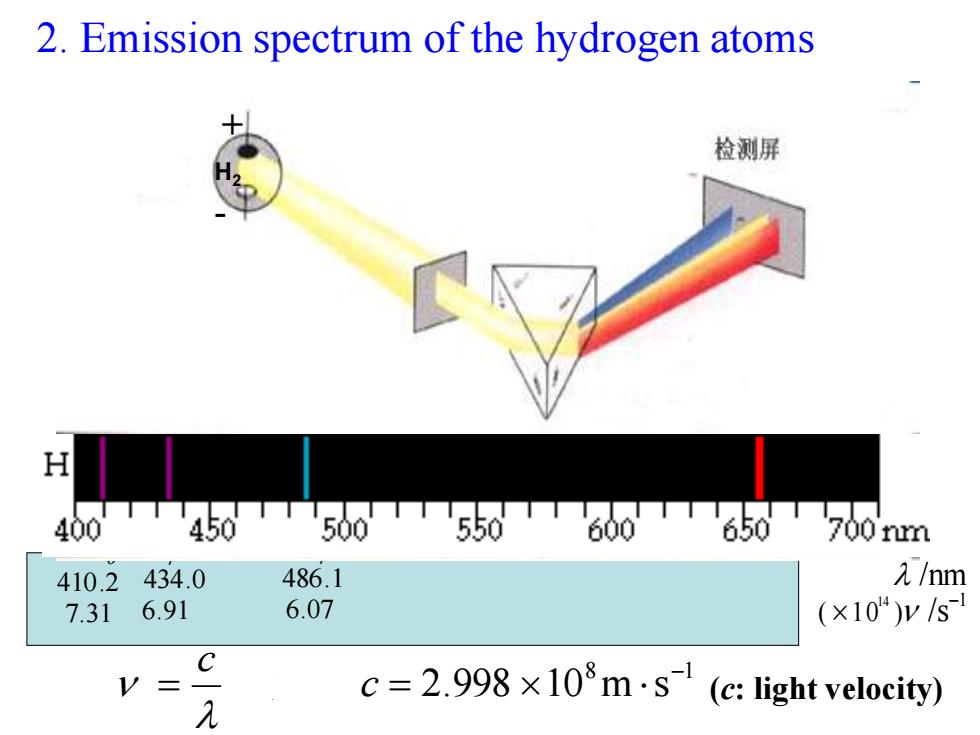
2.Emission spectrum of the hydrogen atoms 检测屏 H 0TTT450TT500550600T650700nm 40 410.2434.0 486.1 元/nm 7.31 6.91 6.07 (×10)y/s C V= 几 c=2.998×108m·s(c:light velocity)
2. Emission spectrum of the hydrogen atoms 8 1 2.998 10 m s c c 光速 Hα 656.3 4.57 Hβ 486.1 6.07 Hγ 434.0 6.91 Hδ 410.2 7.31 /nm1 ( 10 ) /s 14 H2 + - (c: light velocity)