
The principle of multiple equilibria K=(Ke2k9=0.672×0.051=0.023 Conclusion: if a reaction equation is obtained by a linear combination of a couple of reaction equations,the reaction equilibrium constant of the resulting combined reaction is equal to the product /or quotient of the participating reactions'reaction equilibrium constant. -----a principle of multi-equilibria 结论:如果多个反应的计量式经过线性组合得到一个总的化学计量式,则总反应 的标准平衡常数等于各反应的平衡常数之积或商—多重平衡原理
The principle of multiple equilibria Conclusion: if a reaction equation is obtained by a linear combination of a couple of reaction equations, the reaction equilibrium constant of the resulting combined reaction is equal to the product /or quotient of the participating reactions’ reaction equilibrium constant. ------a principle of multi-equilibria 结论: 如果多个反应的计量式经过线性组合得到一个总的化学计量式,则总反应 的标准平衡常数等于各反应的平衡常数之积或商⎯⎯多重平衡原理. = · = 0.67 K3 K 2×0.051=0.023 1 K2 2 ( )
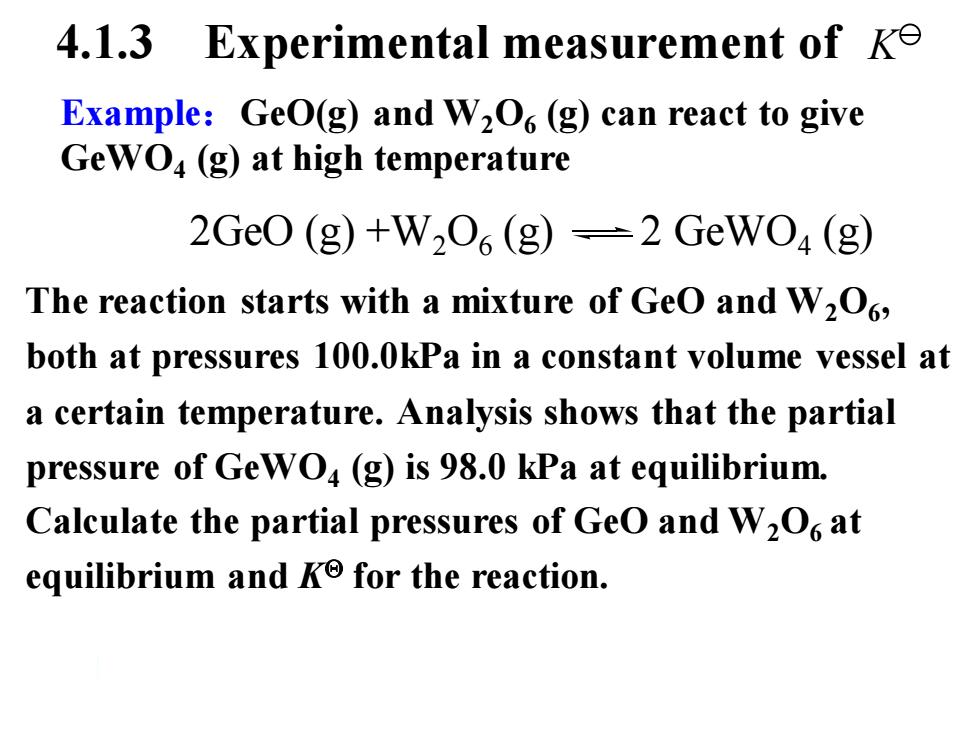
4.1.3 Experimental measurement of ke Example:GeO(g)and W2O6(g)can react to give GewO4(g)at high temperature 2Ge0(g)+W206(g)=2GeWO4(g) The reaction starts with a mixture of GeO and W2O, both at pressures 100.0kPa in a constant volume vessel at a certain temperature.Analysis shows that the partial pressure of GeWO4(g)is 98.0 kPa at equilibrium. Calculate the partial pressures of GeO and W2O at equilibrium and Ko for the reaction
Example:GeO(g) and W2O6 (g) can react to give GeWO4 (g) at high temperature 4.1.3 Experimental measurement of The reaction starts with a mixture of GeO and W2O6 , both at pressures 100.0kPa in a constant volume vessel at a certain temperature. Analysis shows that the partial pressure of GeWO4 (g) is 98.0 kPa at equilibrium. Calculate the partial pressures of GeO and W2O6 at equilibrium and K for the reaction. 2GeO (g) +W2O6 (g) 2 GeWO4 (g) K
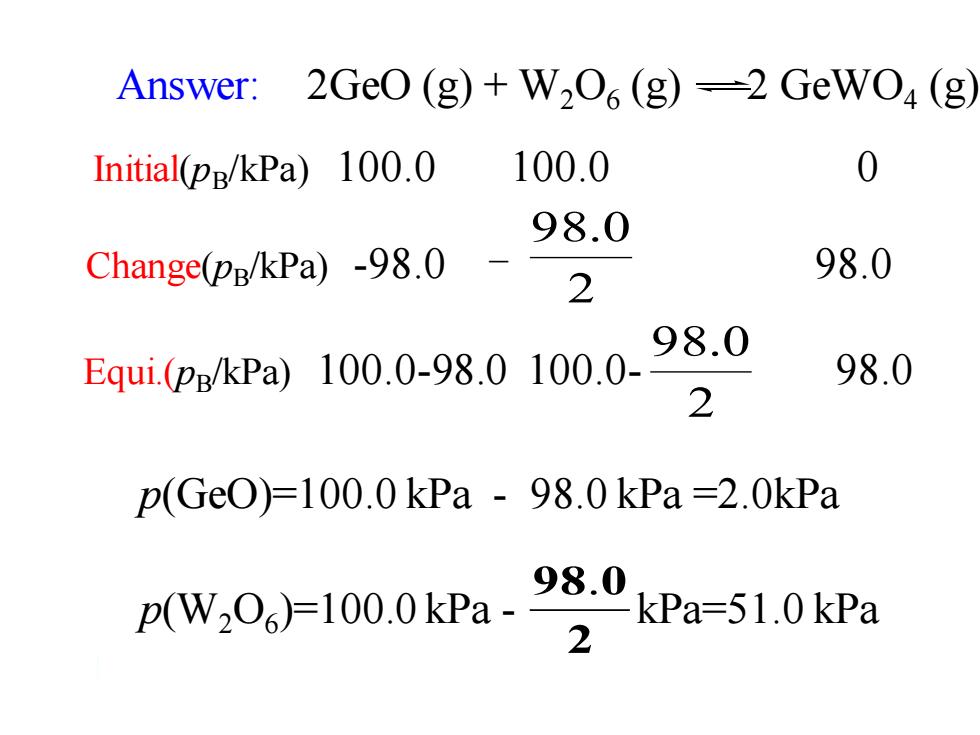
Answer: 2G0(g)+W2O6(g)=2GeW04(g Initial(pg/kPa)100.0 100.0 0 98.0 Change(pg/kPa)-98.0 98.0 2 Equi(P:/KPa)100.0-98.010.0.98.0 98.0 2 p(Ge0)=100.0kPa-98.0kPa=2.0kPa ZkPa-51.0kPa zw,0)100.0kPa98.0
2 98.0 p(W2O6 )=100.0 kPa - kPa=51.0 kPa p(GeO)=100.0 kPa - 98.0 kPa =2.0kPa Answer: 2GeO (g) + W2O6 (g) 2 GeWO4 (g) 2 98.0 Equi.(pB/kPa) 100.0-98.0 100.0- 98.0 Initial(pB/kPa) 100.0 100.0 0 Change(pB/kPa) -98.0 - 98.0 2 98.0
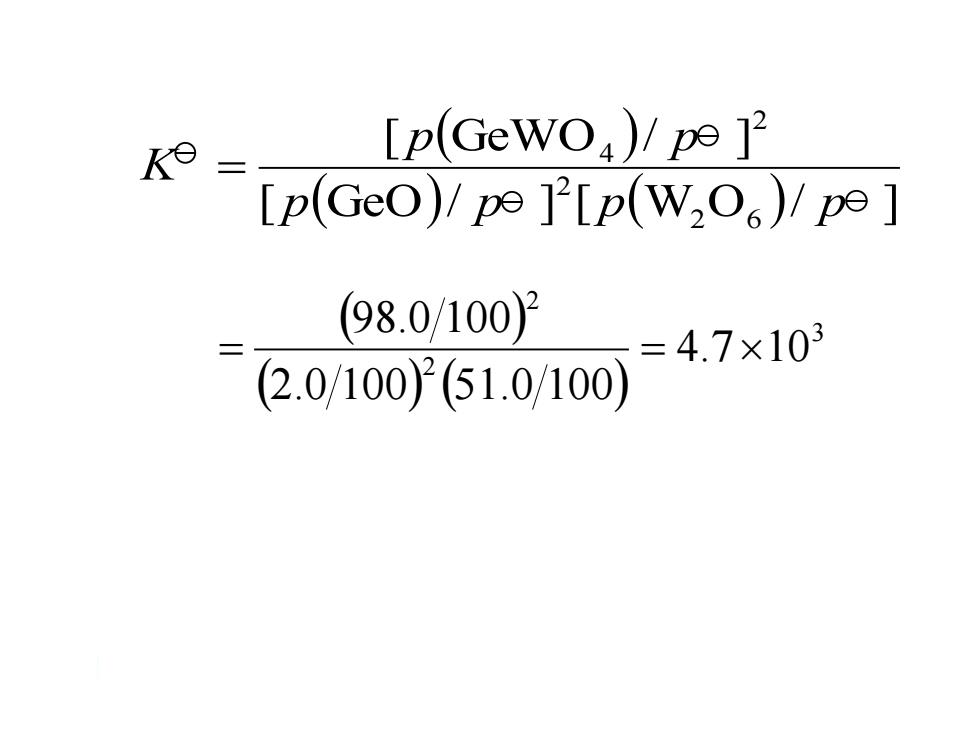
K= [p(GeWO)/pe [p(Geo)!pe P[p(W2O)/pe (98.0/1002 =4.7×103 (2.0/1002(51.0/100)
( ) ( ) ( ) 3 2 2 4.7 10 2.0 100 51.0 100 98.0 100 = = ( ) [ (GeO)/ ] [ (W O )/ ] [ GeWO / ] 2 6 2 2 4 p p p p p p K =
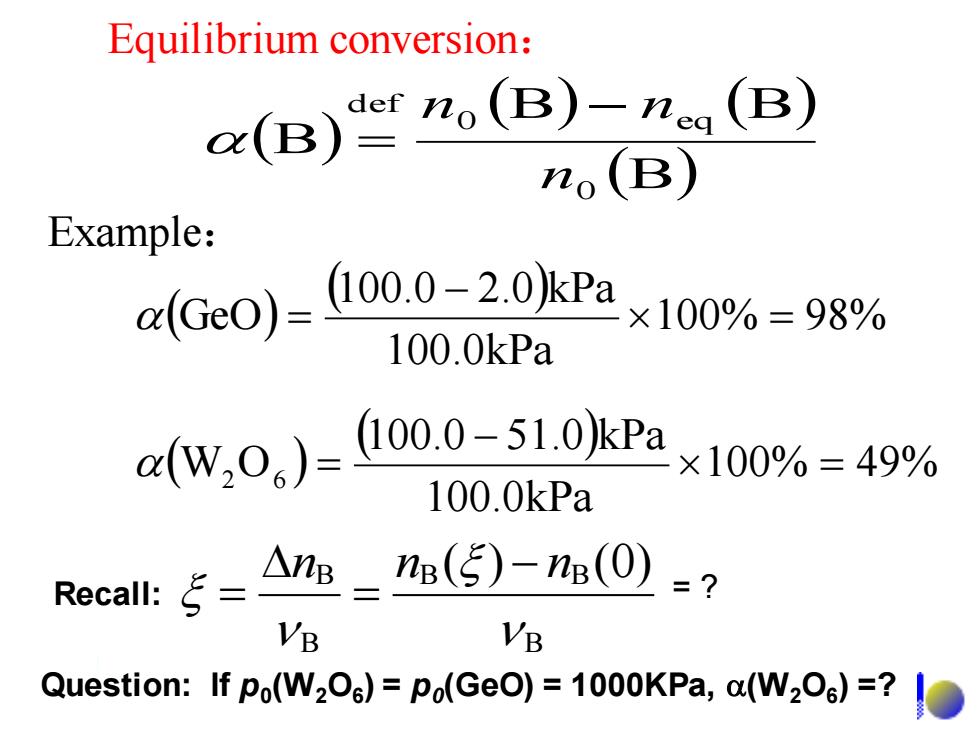
Equilibrium conversion: (B)B)(B) o(B) Example: aGc0)=00.0-2.0kPa ×100%=98% 100.0kPa rw.0,)=000_51oPa×100%=49% 100.0kPa RecalI::ξ= △nB-n(5)-n(0)=? VB VB Question:If po(W2O)=Po(GeO)=1000KPa,a(W2O)=?
Equilibrium conversion: ( ) ( ) ( ) (B) B B B 0 0 eq def n n − n = ( ) ( ) 100% 49% 100.0kPa 100.0 51.0 kPa W2 O6 = − = ( ) ( ) 100% 98% 100.0kPa 100.0 2.0 kPa GeO = − = Example: Question: If p0 (W2O6 ) = p0 (GeO) = 1000KPa, (W2O6 ) =? = ? B B B B B ( ) (0) n n − n = Recall: =