11.2.1 Valence Bond Theory The main points of valence bond theory: 1.Central metal ion(M):having empty orbits Ligands(L):having lone electron pairs The metal-ligand bonds are coordination covalent bond M<L. 2.The metal-ligand bonds are formed by hybrid orbits 3.The different types of hybridization determine the structure of coordination compounds
The main points of valence bond theory: 1. Central metal ion(M):having empty orbits Ligands(L) :having lone electron pairs The metal-ligand bonds are coordination covalent bond ML. 2. The metal-ligand bonds are formed by hybrid orbits. 3. The different types of hybridization determine the structure of coordination compounds. 11.2.1 Valence Bond Theory
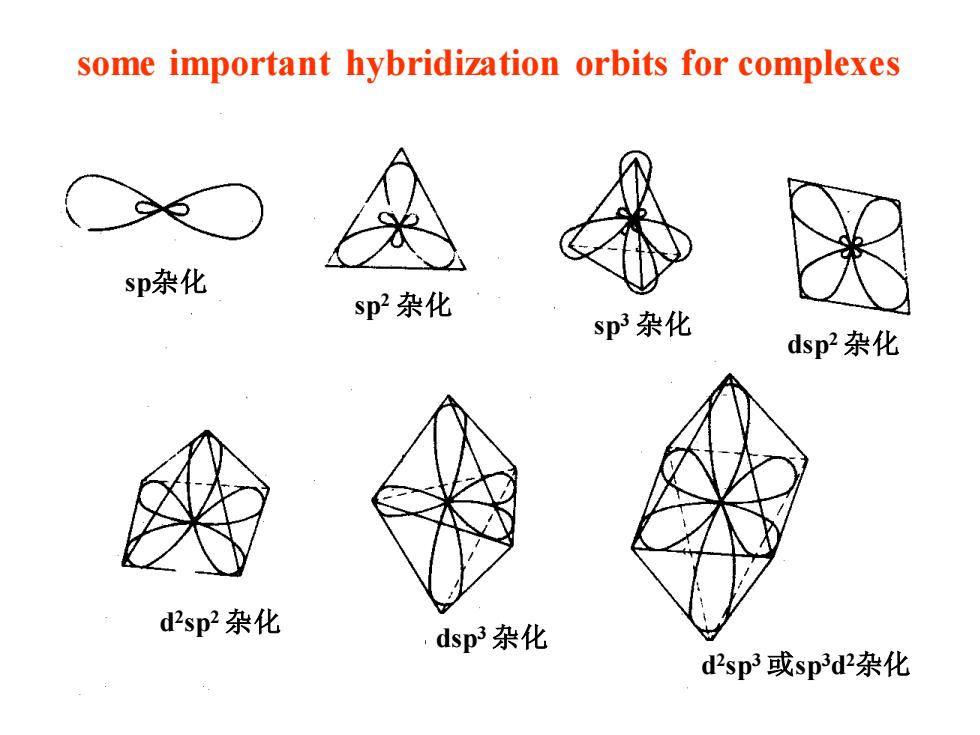
some important hybridization orbits for complexes sp杂化 sp2杂化 sp3杂化 dsp2杂化 d2sp2杂化 dsp3杂化 d2sp3或sp3d2杂化
some important hybridization orbits for complexes sp杂化 sp2 杂化 sp3 杂化 dsp2 杂化 d 2 sp2 杂化 dsp3 杂化 d 2 sp3 或sp3d 2杂化

配位数 杂化轨道 所用的轨道 几何构型 实 例 2 sp s、Px 直线形 〔Ag(CN)2J 3 5p2 S、P Py 正三角形 〔CuC,)2- sp3 S、Px、Py、Pz 正四面体 Ni(C0)4〕 dsp2 dx-yS、px、py 平面正方形 [Ni(CN)4)2- dsp dz2、5、px、py、p 三角双锥体 CFe(cos〕 5 d2sp2 d-ya、d:2、s、pxpy 四方锥体 d2sp dx-yd,2、5、px八Py八p 正八面体 Fe(CN)6)3- 6 spd s、px、p,、p、dx-y小d:2 正八面体 〔FeF6〕3-