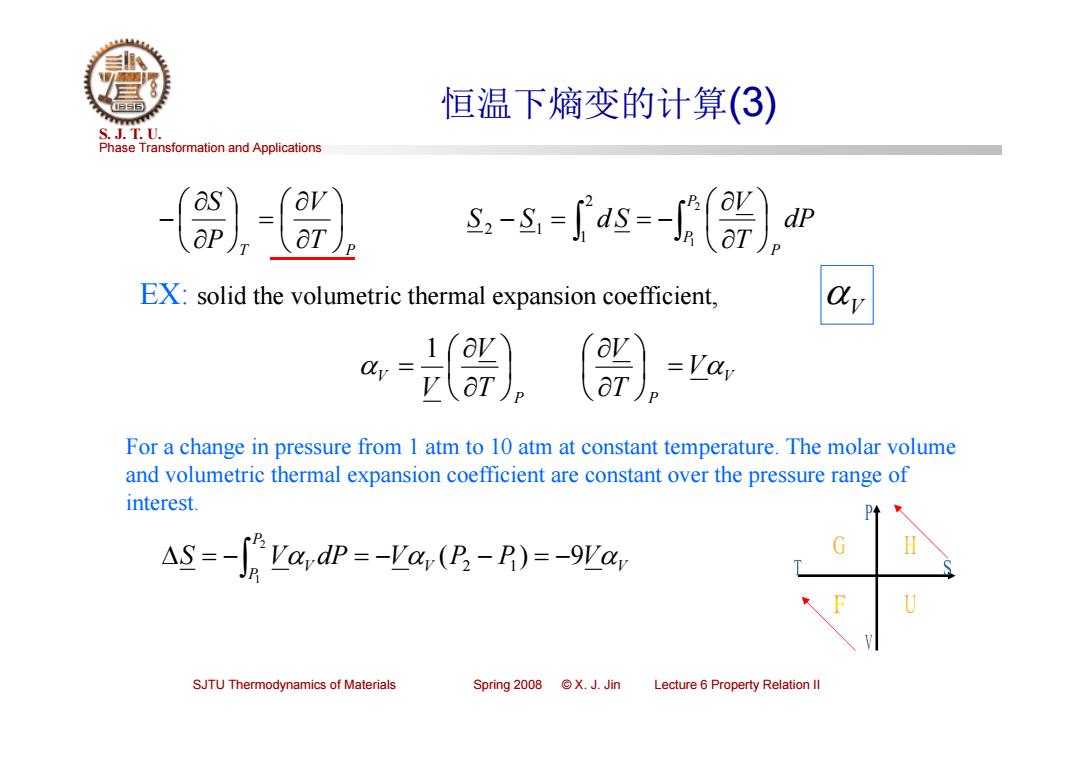
恒温下熵变的计算(③) S.J.T.0. Phase Transformation and Applications dp ap s-s-s=-〔 EX:solid the volumetric thermal expansion coefficient, For a change in pressure from 1 atm to 10 atm at constant temperature.The molar volume and volumetric thermal expansion coefficient are constant over the pressure range of interest. AS=-f:VardP--Var(P-R)=-9Var H SJTU Thermodynamics of Materials Spring2o08©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II 恒温下熵变的计算(3) T T P V P S 21 21 2 1 PP P dP TV S S d S EX: solid the volumetric thermal expansion coefficient, For a change in pressure from 1 atm to 10 atm at constant temperature. The molar volume and volumetric thermal expansion coefficient are constant over the pressure range of interest. V V P P S VV dP V (P2 P1) 9V 2 1 V V P P V V TV TV V 1

恒温下熵变的计算(4) S.J.T.0. Phase Transformation and Applications EX:copper, ,=16.7×106K-1 =13 InV=3Inl ).-2) ay 3ar For a change in pressure from 1 atm to 10 atm at constant temperatrure AS=-fVardP--Vav(P-P)=-9RVar--27RVa H =-3.24×10-4J/mol.K) △S=-19.14J/molK) for an ideal gas SJTU Thermodynamics of Materials Spring 2008 ©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II 恒温下熵变的计算(4) For a change in pressure from 1 atm to 10 atm at constant temperatrure J mol K S V dP V V P P PV V PV l P P V 3.24 10 / ( ) 9 27 4 2 1 1 1 2 1 EX: copper, 6 1 16.7 10 l K l dl V dV V l lnV 3ln l 3 3 V l P T l dT l dV V 3 1 3 S 19.14 J / mol K for an ideal gas