
9.2 VSEPR theory The strength of repulsions are in the order: lone pair-lone pair lone pair-atom>atom-atom Two pairs A pair of lone of lone electrons electron 104.5 107.3 Ammonia molecule Water molecule one pairs should in the positions to minimize the repulsion between the electron domains
- The are in the order: - - strength of repulsions lone pair lone pair lone pair atom atom atom > > 9.2 VSEPR theory Lone pairs should in the positions to minimize the repulsion between the electron domains
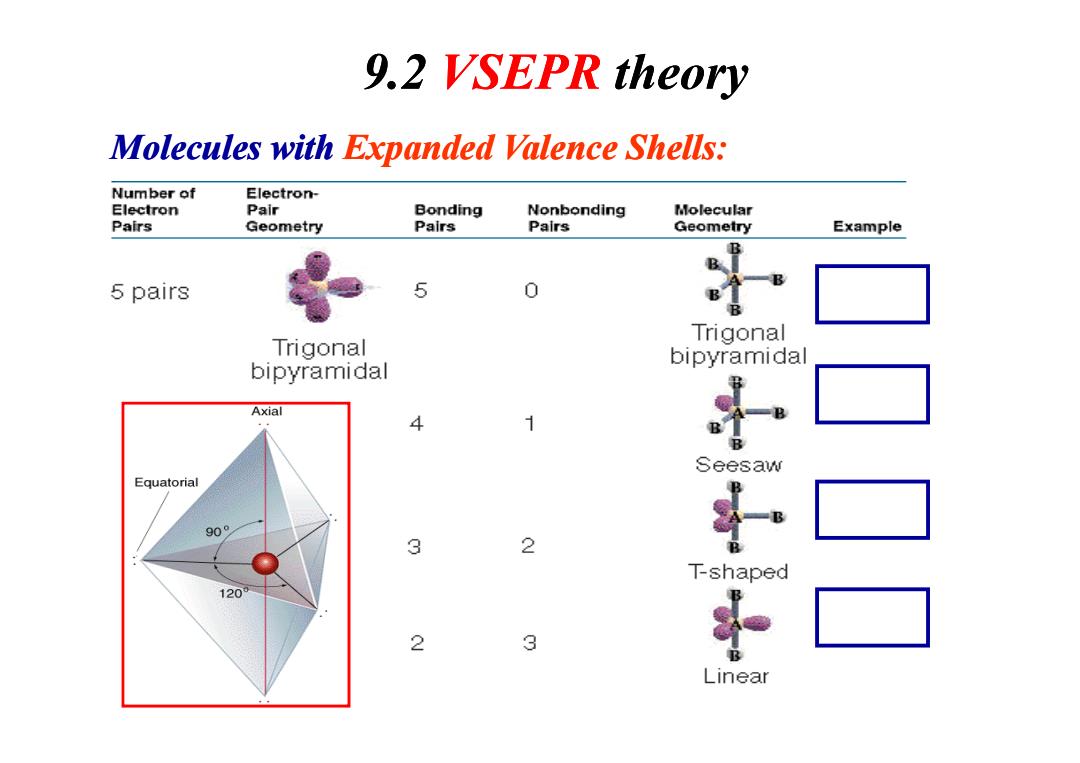
9.2 VSEPR theory Molecules with Expanded Valence Shells: Number of Electron- Electron Pair Bonding Nonbonding Molecular Pairs Geometry Pairs Pairs Geometry Example B 5 pairs 5 0 B Trigonal Trigonal bipyramidal bipyramidal Axial 4 1 B B Seesaw Equatorial 90° 3 2 T-shaped 120 2 3 Linear
Molecules with Expanded Valence Shells: 9.2 VSEPR theory
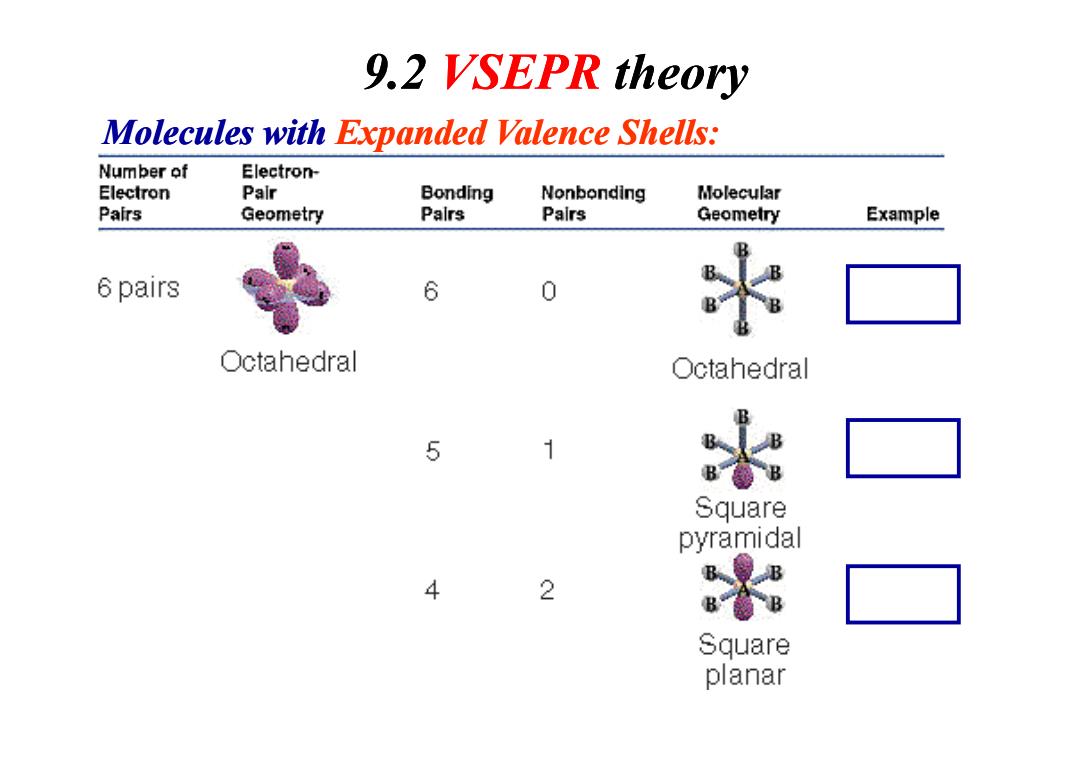
9.2 VSEPR theory Molecules with Expanded Valence Shells: Number of Electron- Electron Palr Bonding Nonbonding Molecular Pairs Geometry Palrs Palrs Geometry Example 6 pairs 6 Octahedral ○ctahedral 5 1 Square pyramidal B 4 2 Square planar
9.2 VSEPR theory Molecules with Expanded Valence Shells:

Predicting Molecular Geometries Step 1:Draw the Lewis structure Step 2:Count the total number ofelectron pairs around the central atom Step 3:Arrange the electron pairs in one of the above geometries to minimize e-e repulsion (Multiple bonds count as one bonding pair) Step 4:determine molecular geometry NH, → H-N-H H Lewis structure H Electron-pair geometry Molecular geometry (tetrahedral) (trigonal pyramidal)
Step 1: Draw the Lewis structure Step 2: Count the total number of electron pairs around the central atom Step 3: Arrange the electron pairs in one of the above geometries to minimize e--e- repulsion (Multiple bonds count as one bonding pair) Predicting Molecular Geometries Step 4: determine molecular geometry NH3
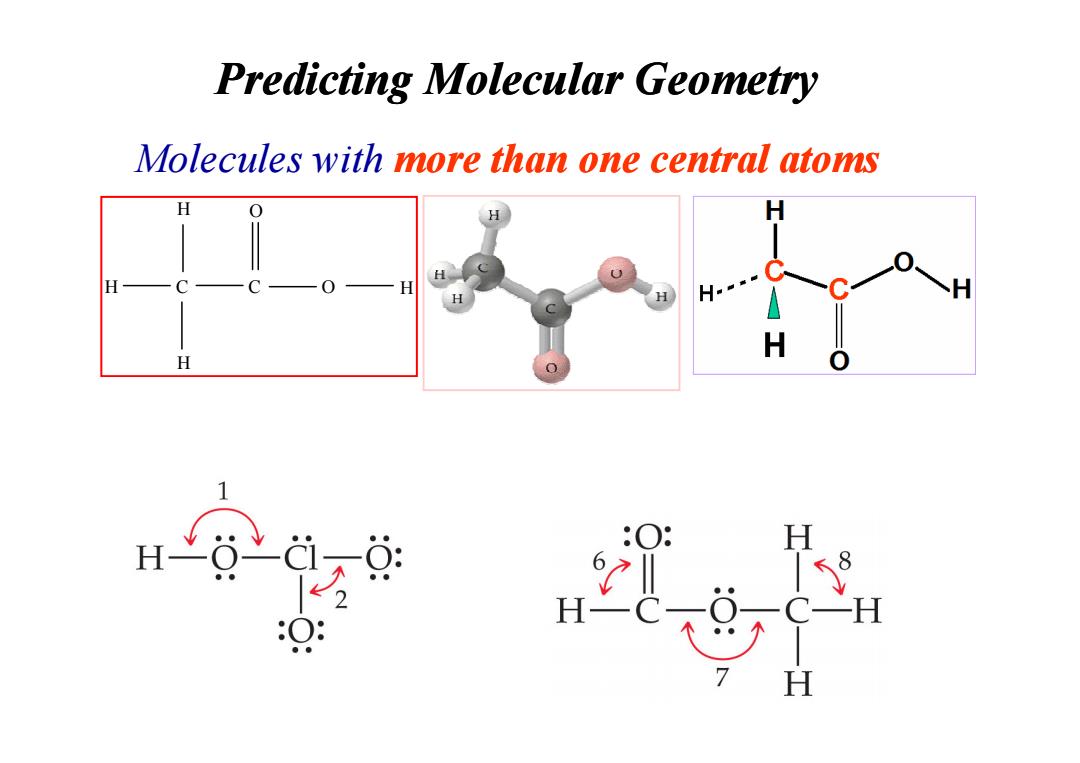
Predicting Molecular Geometry Molecules with more than one central atoms H H H H H H H-- H H 0 H H
Predicting Molecular Geometry Molecules with more than one central atoms H H C O O H H C