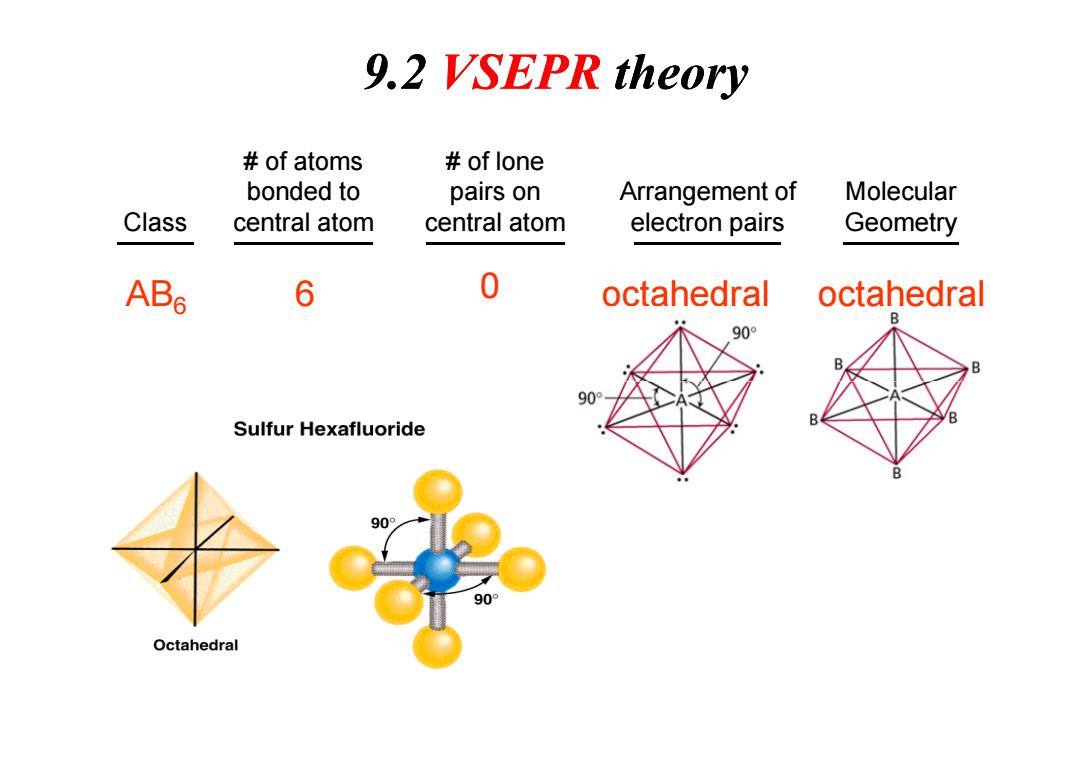
9.2 VSEPR theory of atoms of lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry ABs 6 0 octahedral octahedral 90° 90 B Sulfur Hexafluoride 90° 90° Octahedral
Class # of atoms bonded to central atom # of lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB6 6 0 octahedral octahedral 9.2 VSEPR theory

TABLE 9.1 Electron-Domain Geometriesas a Function of the Number of of Electron Domains Number of Arrangement of Electron-Domain Predicted Electron Domains Electron Domains Geometry Bond Angles 2 Linear 180° 3 Trigonal 120° planar both bonding and 4 Tetrahedral 109.5° nonbonding electron pairs 5 Trigonal- 120° bipyramidal 90° 6 Octahed ral 90° 180°
both bonding and nonbonding electron pairs

9.2 VSEPR theory Predicting Molecular Geometries: Total Electron- Electron Pair Bonding Nonbonding Molecular Pairs Geometry Pairs Pairs Geometry Example 2 0 BA✉B 2 pairs Linear Linear 3 pairs 3 0 Trigonal planar Trigonal planar 2 Bent
Predicting Molecular Geometries: 9.2 VSEPR theory Trigonal planar

9.2 VSEPR theory Predicting Molecular Geometries: NO? 1 +1 A single unpaired electron on the central atom also has the high electron density,and is treated like a lone pair
Predicting Molecular Geometries: NO? 9.2 VSEPR theory A single unpaired electron on the central atom also has the high electron density, and is treated like a lone pair
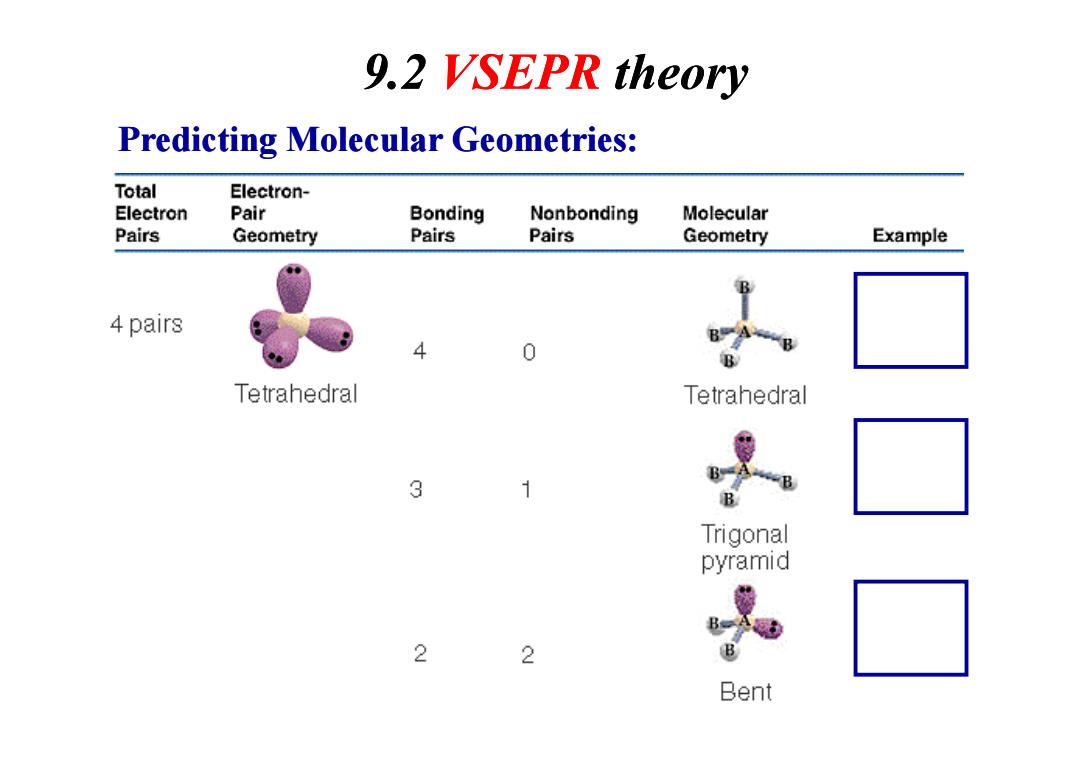
9.2 VSEPR theory Predicting Molecular Geometries: Total Electron- Electron Pair Bonding Nonbonding Molecular Pairs Geometry Pairs Pairs Geometry Example 4 pairs 4 0 Tetrahedral Tetrahedral 3 1 B Trigonal pyramid B 2 2 B Bent
Predicting Molecular Geometries: 9.2 VSEPR theory