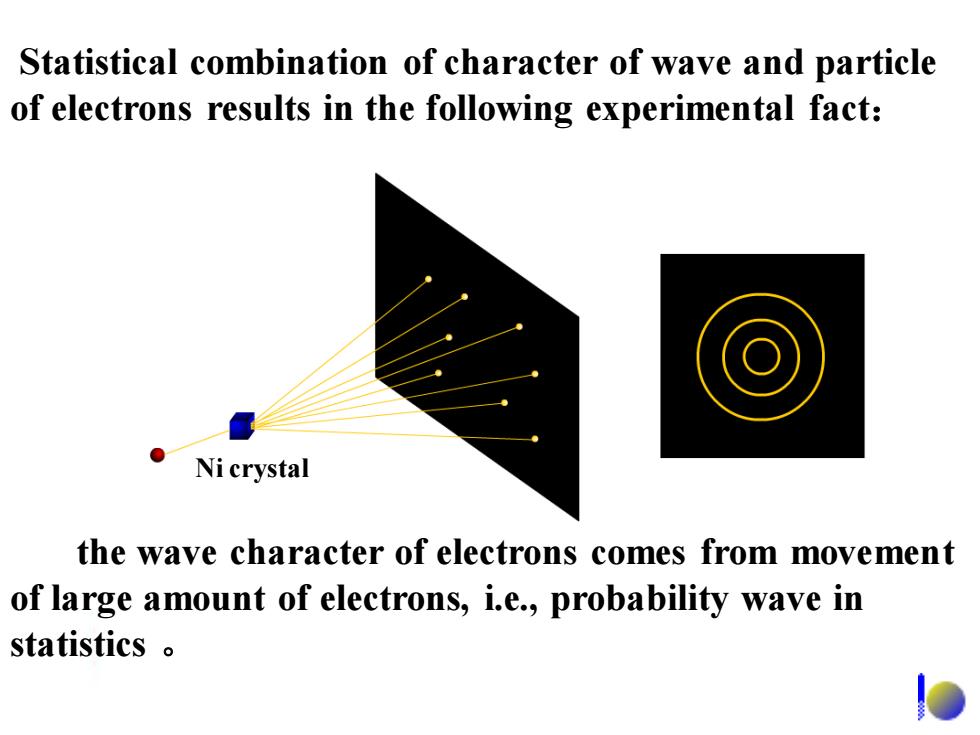
Statistical combination of character of wave and particle of electrons results in the following experimental fact: Ni crystal the wave character of electrons comes from movement of large amount of electrons,i.e.,probability wave in statistics
Statistical combination of character of wave and particle of electrons results in the following experimental fact: the wave character of electrons comes from movement of large amount of electrons, i.e., probability wave in statistics 。 Ni crystal
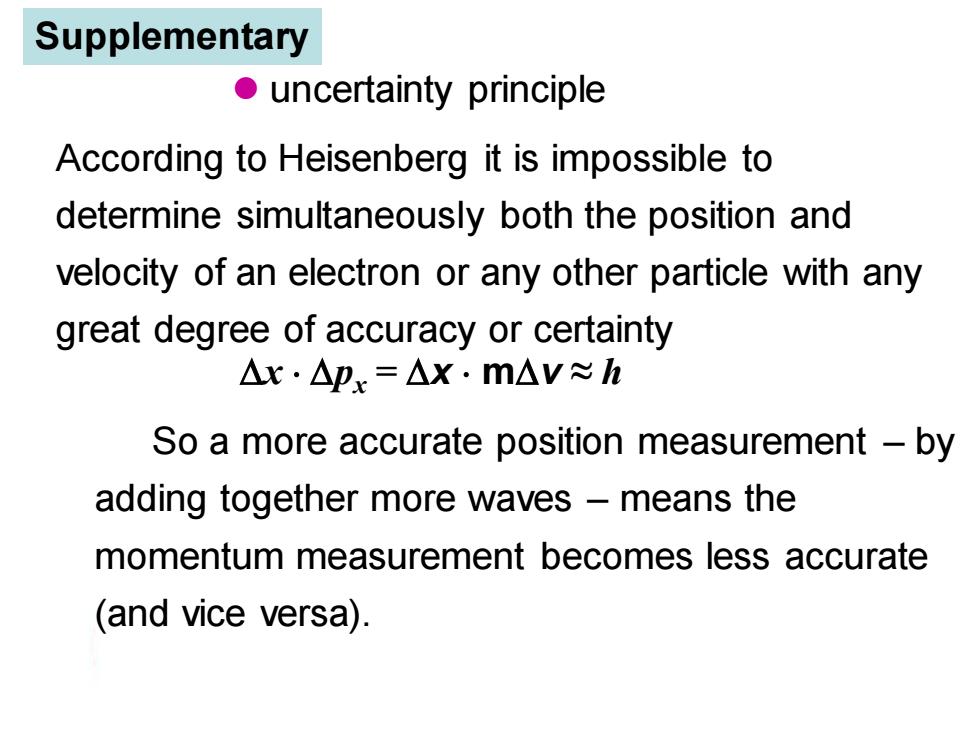
Supplementary uncertainty principle According to Heisenberg it is impossible to determine simultaneously both the position and velocity of an electron or any other particle with any great degree of accuracy or certainty △x·△px=△x.m△V≈h So a more accurate position measurement -by adding together more waves -means the momentum measurement becomes less accurate (and vice versa)
According to Heisenberg it is impossible to determine simultaneously both the position and velocity of an electron or any other particle with any great degree of accuracy or certainty So a more accurate position measurement – by adding together more waves – means the momentum measurement becomes less accurate (and vice versa). ⚫ uncertainty principle x px = x mv ≈ h Supplementary

8.1.3 Schrodinger equation and quantum numbers 1.Schrodinger equation 02Ψ,02Ψ,02Ψ x2 0y2 0z2 Ψ:a wave function E:the overall energy of an electron I:the potential energy m:the mass of an electron h:Planck's constant x,y,the cartesian coordinate s
1.SchrÖdinger equation 8.1.3 Schrödinger equation and quantum numbers x, y,z:the cartesian coordinate s V: the potential energy E: the overall energy of an electron Ψ:a wave function m: the mass of an electron h:Planck's constant (E V) Ψ h m z Ψ y Ψ x Ψ = − − + + 2 2 2 2 2 2 2 2 8 π

2.Quantum numbers 平,n1,m(x,y,2) 1 The principle quantum number,n n=1,2,3,... 2The angular momentum quantum number,l 1=0,1,2,.n-1 3 The magnetic quantum number,m m=+l,..0..,-l 4The electron spin quantum number,m 1 ms m=- 2
2. Quantum numbers ① The principle quantum number, n l = 0,1,2,...n −1 ③ The magnetic quantum number, m ④ The electron spin quantum number, ms , 2 1 ms = m = +l,......0......,−l 2 1 ms = − ②The angular momentum quantum number, l n=1, 2, 3,…… ( , , ) , , Ψ x y z n l m
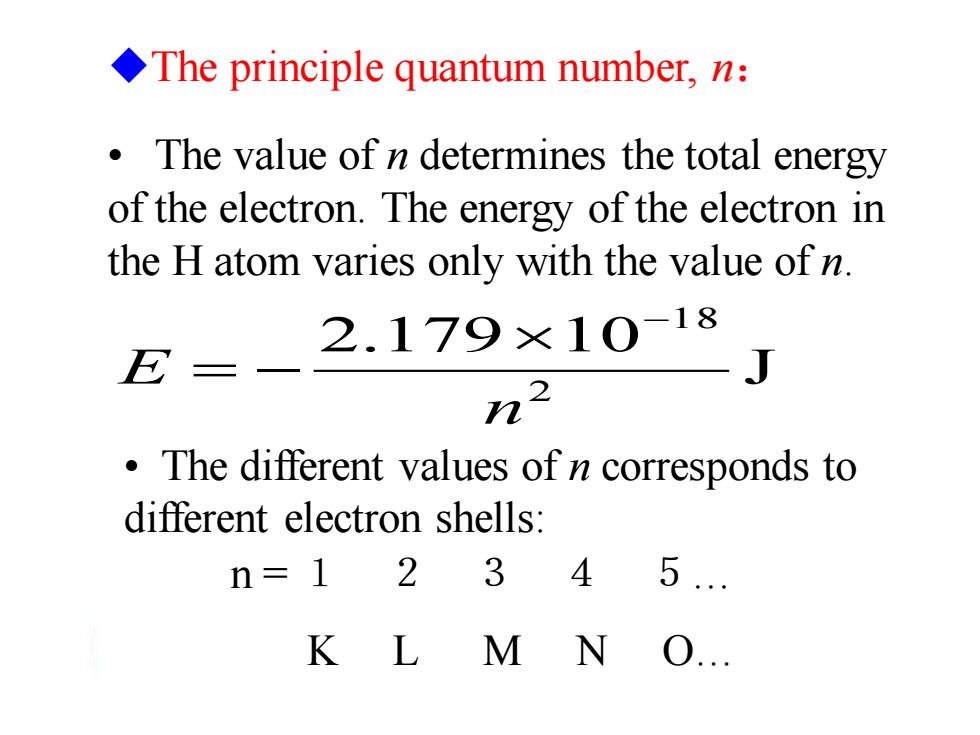
The principle quantum number,n: The value of n determines the total energy of the electron.The energy of the electron in the H atom varies only with the value of n. E 2.179×10-18 三 J 222 The different values of n corresponds to different electron shells: n=12 3 4 5. K L M N O
• The value of n determines the total energy of the electron. The energy of the electron in the H atom varies only with the value of n. J 2.179 10 2 1 8 n E − = − • The different values of n corresponds to different electron shells: ◆The principle quantum number, n: n = 1 2 3 4 5… K L M N O…