
Review Geometrical Arrangements Characteristic of Hybrid Orbital Sets Atomic Hybrid 构型 Orbital Set Orbital Set Geometry Examples Geometry 平面三角形 Trigonal bipyramidal, d Five sp PFs,SF4,BrF3 Seesaw跷跷板 sp'd T shape T-形 120° The elements in period Trigonal 3 or beyond with empty bipyramidal d orbitals 八面体 Octahedral, sypp,d Six sp2 SF6,CIFs,XeF4,PF 90 Square pyramidal sp'd- Square planar octahedral 四角锥 平面正方形
Review 3 sp d Trigonal Geometry Trigonal bipyramidal, Seesaw T shape 跷跷板 平面三角形 构型 T-形 The elements in period 3 2 sp d octahedral Trigonal bipyramidal Octahedral, Square pyramidal Square planar 八面体 四角锥 平面正方形 The elements in period 3 or beyond with empty d orbitals
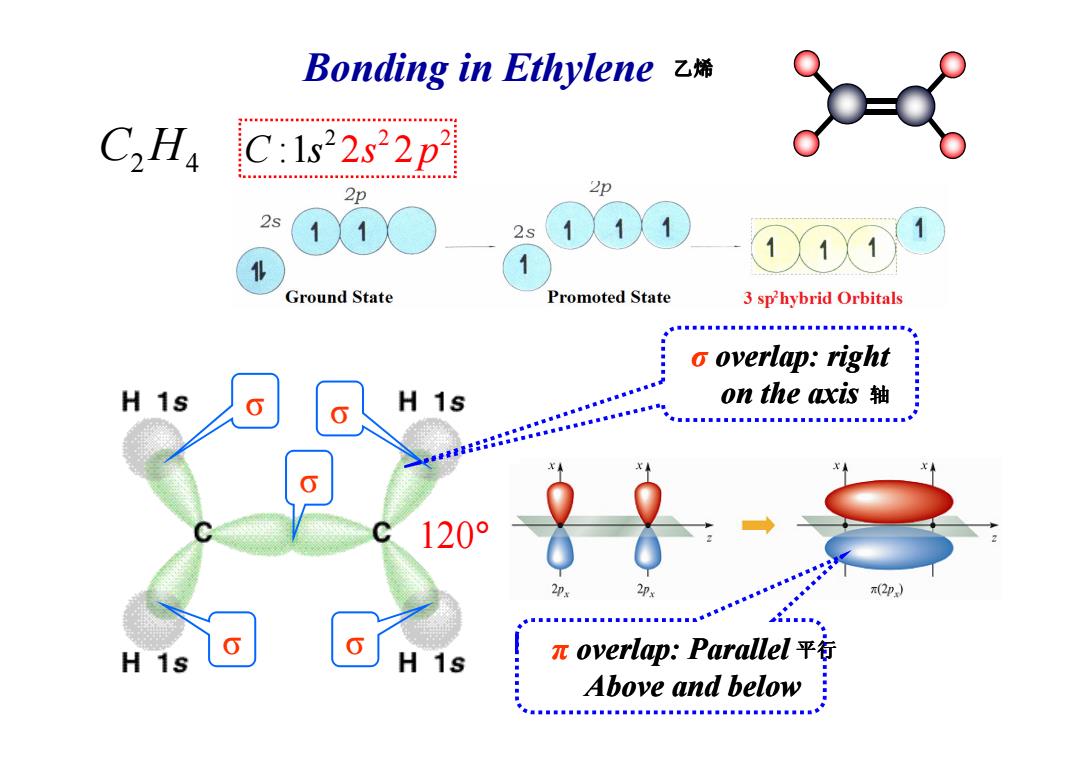
Bonding in Ethylene乙烯 C2 Ha C:1s22s22p 2p 2p 2s 2s 11 Ground State Promoted State 3 sphybrid Orbitals o overlap:right H 1s 222.0aa00 H 1s on the axis轴 120° 2px x(2p) 年■■■■■■■■■■■■■■■■■g。装装地着 H 1s H 1s πoverlap:Parallel平行 Above and below c.........c...s
Bonding in Ethylene C H2 4 2 2 2 C s :1 2 2 s p σ overlap: right on the axis 乙烯 σ σ σ σ σ 120 ° on the axis π overlap: Parallel Above and below 平行 轴
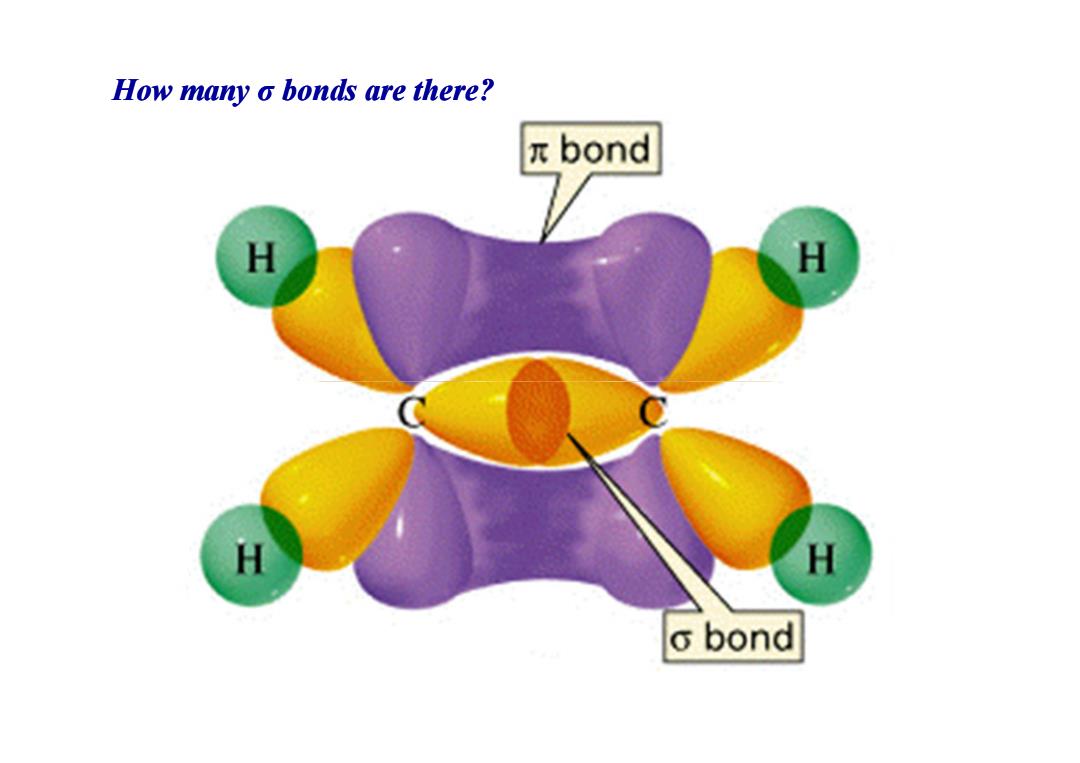
How many o bonds are there? πbond H H H H o bond
How many How many σ bonds are there? bonds are there?
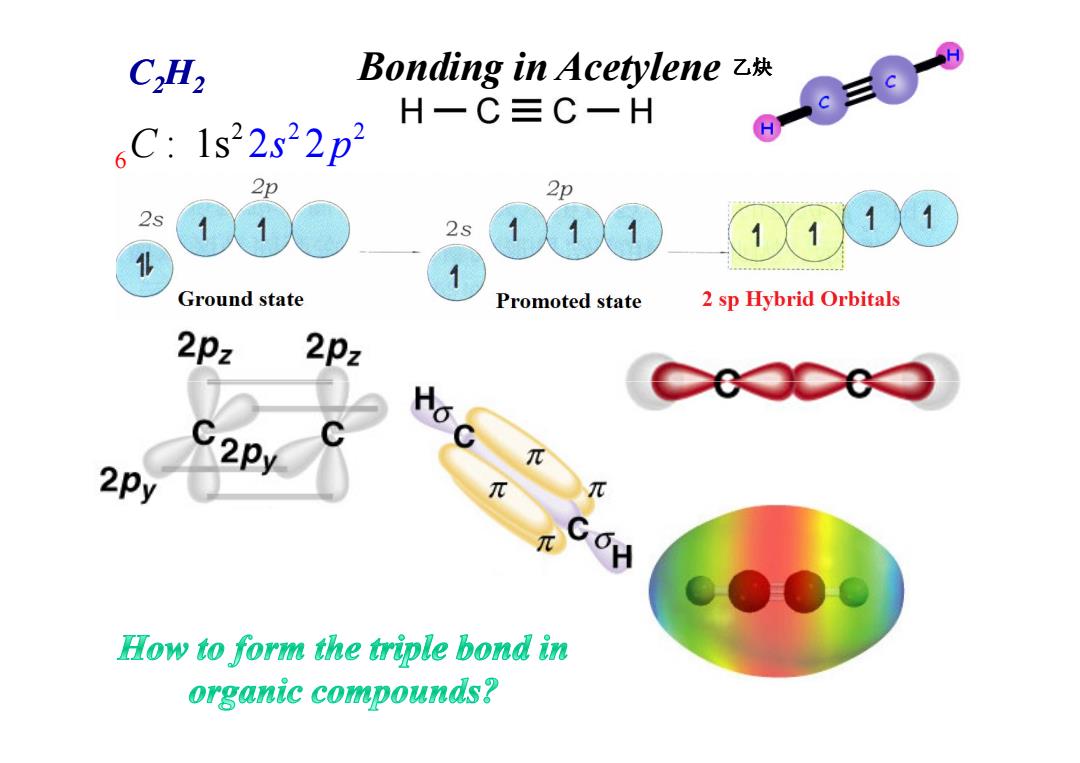
C2H2 Bonding in Acetylene z块 H一C三C一H ,C:1s22s22p2 2p 2p 2s 2s 1 1 人1X 1 Ground state Promoted state 2 sp Hybrid Orbitals 2pz 2pz C2Py 6 2Py How to form the triple bond in organic compounds?
Bonding in Acetylene 2 6 2 2 C : 1s 2s p 2 C2H2 乙炔
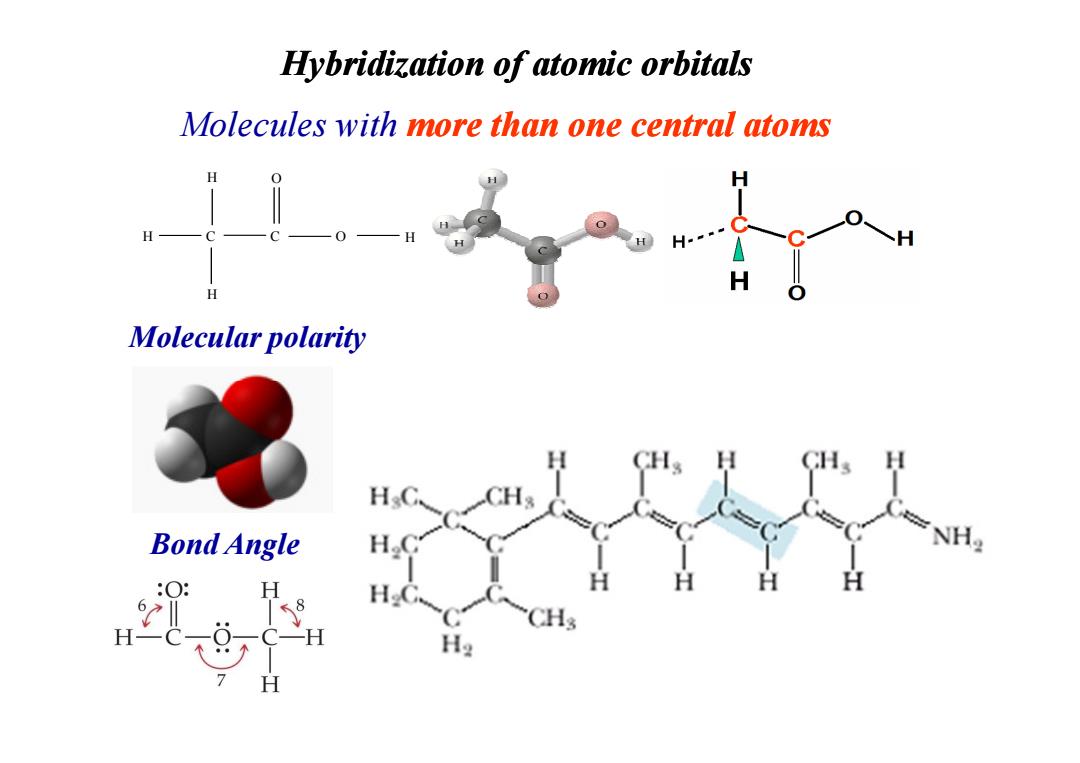
Hybridization of atomic orbitals Molecules with more than one central atoms H H C H H-= H H Molecular polarity CH H Bond Angle NH2 :O H ≤8 H2
Hybridization of atomic Hybridization of atomic orbitals orbitals Molecules with more than one central atoms H H C O O H H C Molecular polarity Bond Angle