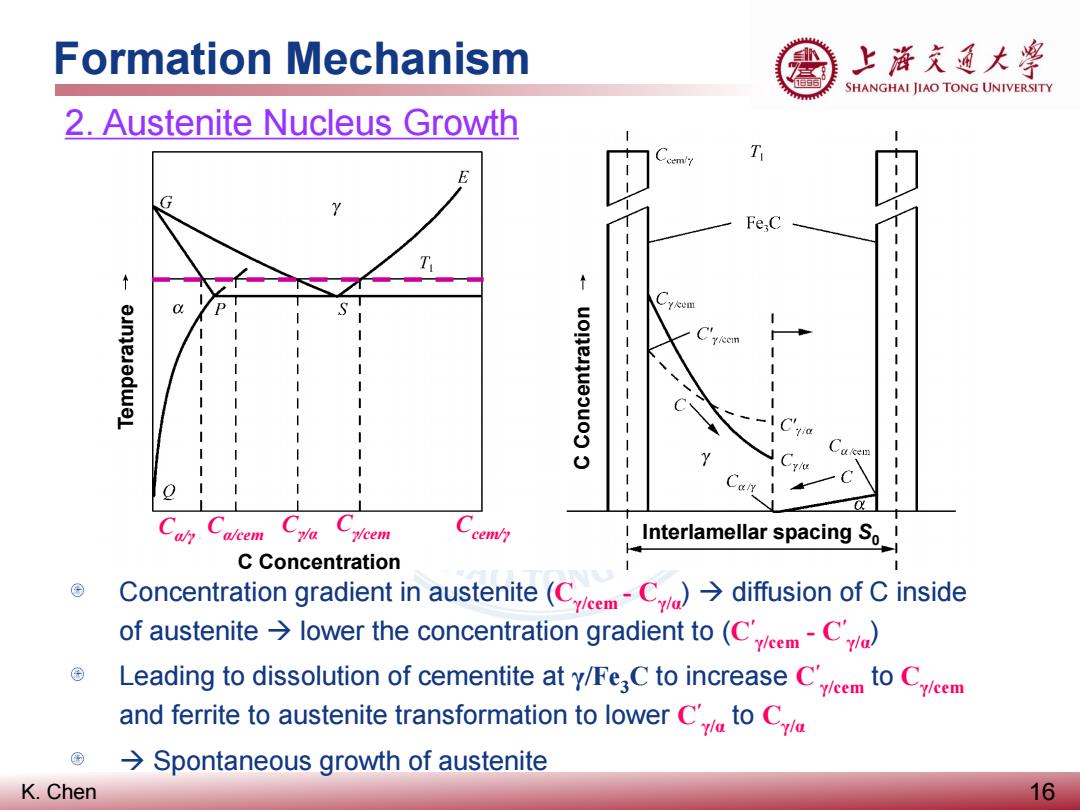
Formation Mechanism 上海充通大粤 SHANGHAI JIAO TONG UNIVERSITY 2.Austenite Nucleus Growth Fe,C a/cem ycem cem/y Interlamellar spacing So! C Concentration Concentration gradient in austenite (CCdiffusion of C inside of austenite lower the concentration gradient to(Cem-C Leading to dissolution of cementite at y/Fe C to increase C to Cem and ferrite to austenite transformation to lower Cto C Spontaneous growth of austenite K.Chen 16
K. Chen Formation Mechanism 16 2. Austenite Nucleus Growth Cα/γ Cα/cem Cγ/α Cγ/cem Ccem/γ Concentration gradient in austenite (Cγ/cem - Cγ/α) diffusion of C inside of austenite lower the concentration gradient to (C′ γ/cem - C′ γ/α) Leading to dissolution of cementite at γ/Fe3C to increase C′ γ/cem to Cγ/cem and ferrite to austenite transformation to lower C′ γ/α to Cγ/α Spontaneous growth of austenite Temperature C Concentration C Concentration Interlamellar spacing S0

Formation Mechanism 上游充通大兽 SHANGHAI JIAO TONG UNIVERSITY 2.Austenite Nucleus Growth Fe,C ycem cem/y Interlamellar spacing So_ C Concentration Diffusion of C inside of ferrite due to the carbon concentration gradient (Cc-C)also induces the growth of austenite Austenite nucleation at a/Fe C interfaces results in the concentration gradient in austenite,which becomes the driving force for the austenite nucleus growth K.Chen 17
K. Chen Formation Mechanism 17 2. Austenite Nucleus Growth Cα/γ Cα/cem Cγ/α Cγ/cem Ccem/γ Diffusion of C inside of ferrite due to the carbon concentration gradient (Cα/cem-Cα/γ) also induces the growth of austenite Austenite nucleation at α/Fe3C interfaces results in the concentration gradient in austenite, which becomes the driving force for the austenite nucleus growth Temperature C Concentration C Concentration Interlamellar spacing S0
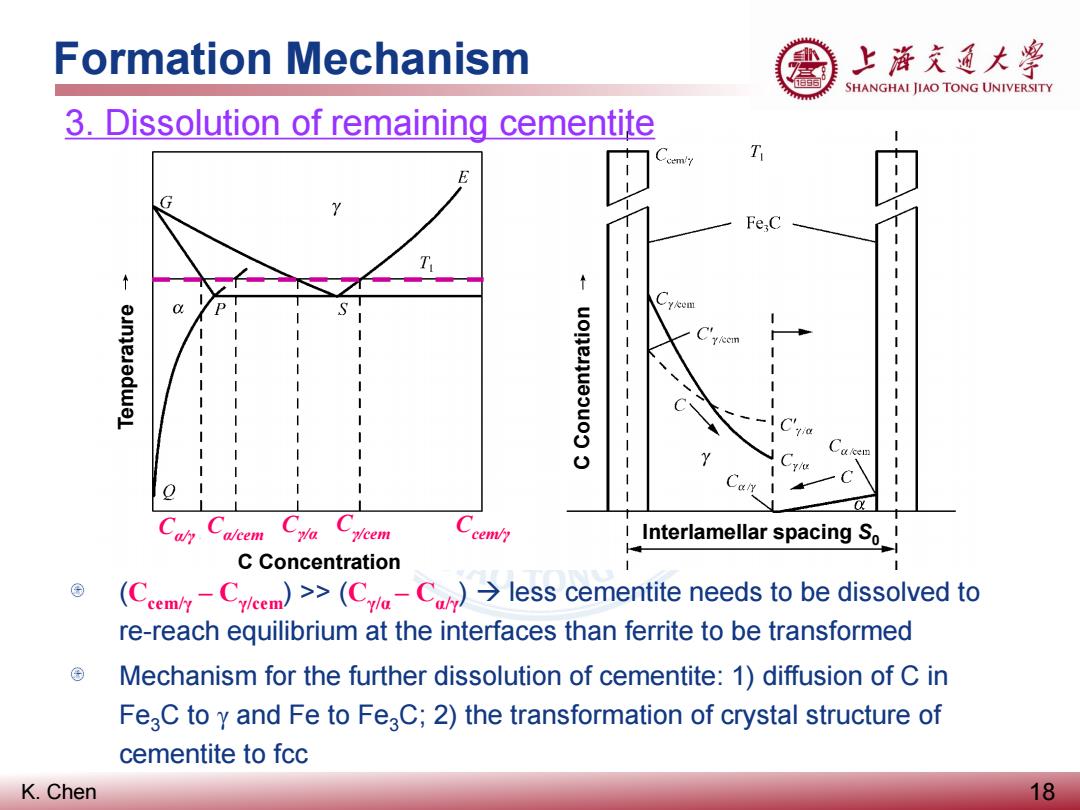
Formation Mechanism 上游充通大兽 SHANGHAI JIAO TONG UNIVERSITY 3.Dissolution of remaining cementite cem/7 T Fe:C O. y/cem cem/y Interlamellar spacing So! C Concentration (Cm-Cieem)>>(C-Cless cementite needs to be dissolved to re-reach equilibrium at the interfaces than ferrite to be transformed Mechanism for the further dissolution of cementite:1)diffusion of C in FeaC to y and Fe to FeaC;2)the transformation of crystal structure of cementite to fcc K.Chen 18
K. Chen Formation Mechanism 18 3. Dissolution of remaining cementite Cα/γ Cα/cem Cγ/α Cγ/cem Ccem/γ (Ccem/γ – Cγ/cem) >> (Cγ/α – Cα/γ) less cementite needs to be dissolved to re-reach equilibrium at the interfaces than ferrite to be transformed Mechanism for the further dissolution of cementite: 1) diffusion of C in Fe3C to γ and Fe to Fe3C; 2) the transformation of crystal structure of cementite to fcc Temperature C Concentration C Concentration Interlamellar spacing S0
Formation Mechanism 上游充道大粤 SHANGHAI JIAO TONG UNIVERSITY 4.Homogenization of austenite Carbon distribution in austenite is not homogeneous right after the dissolution of the remaining cementite Heating is still necessary to achieve homogeneity through carbon diffusion w SANGHALIAO TONG UNIVEE 三 K.Chen 19
K. Chen Formation Mechanism 19 4. Homogenization of austenite Carbon distribution in austenite is not homogeneous right after the dissolution of the remaining cementite Heating is still necessary to achieve homogeneity through carbon diffusion

Formation Mechanism 上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Fe3C Undissolyed Fe3C Austenite nucleation Austenite growth Remaining Fe3C Dissolution of Austenite homogenization remaining Fe3C K.Chen 20
K. Chen Formation Mechanism 20 F Fe3C Undissolved Fe3C A Remaining Fe3C A A A Austenite nucleation Austenite growth Dissolution of remaining Fe3C Austenite homogenization