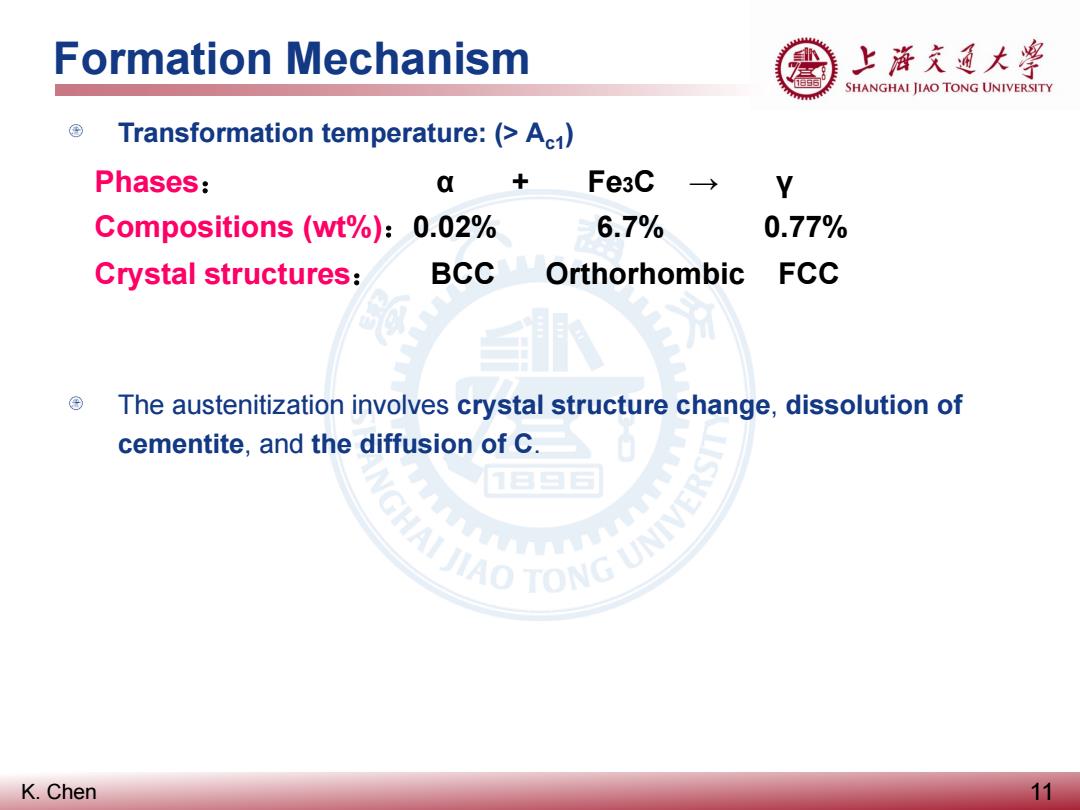
Formation Mechanism 上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Transformation temperature:(>Ac1) Phases: a + Fe3C Y Compositions (wt%):0.02% 6.7% 0.77% Crystal structures: BCC Orthorhombic FCC The austenitization involves crystal structure change,dissolution of cementite,and the diffusion of C. JIAO TONG UNIV 食 K.Chen 11
K. Chen Formation Mechanism 11 Transformation temperature: (> Ac1) The austenitization involves crystal structure change, dissolution of cementite, and the diffusion of C. Phases: α + Fe3C → γ Compositions (wt%):0.02% 6.7% 0.77% Crystal structures: BCC Orthorhombic FCC
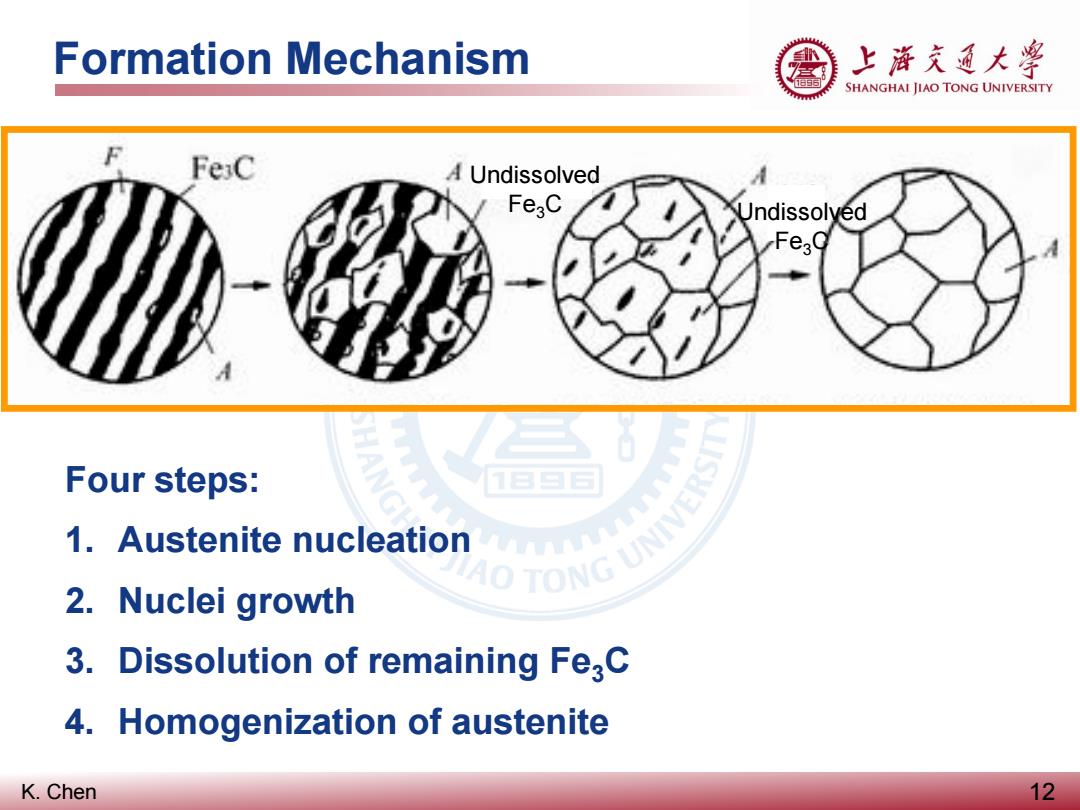
Formation Mechanism 上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY F FeC A Undissolved Fe3C Undissolyed Four steps: 1.Austenite nucleation AO TONG UNIVE 2.Nuclei growth 3.Dissolution of remaining Fe3C 4.Homogenization of austenite K.Chen 12
K. Chen Formation Mechanism 12 Four steps: 1. Austenite nucleation 2. Nuclei growth 3. Dissolution of remaining Fe3C 4. Homogenization of austenite Undissolved Fe3C Undissolved Fe3C
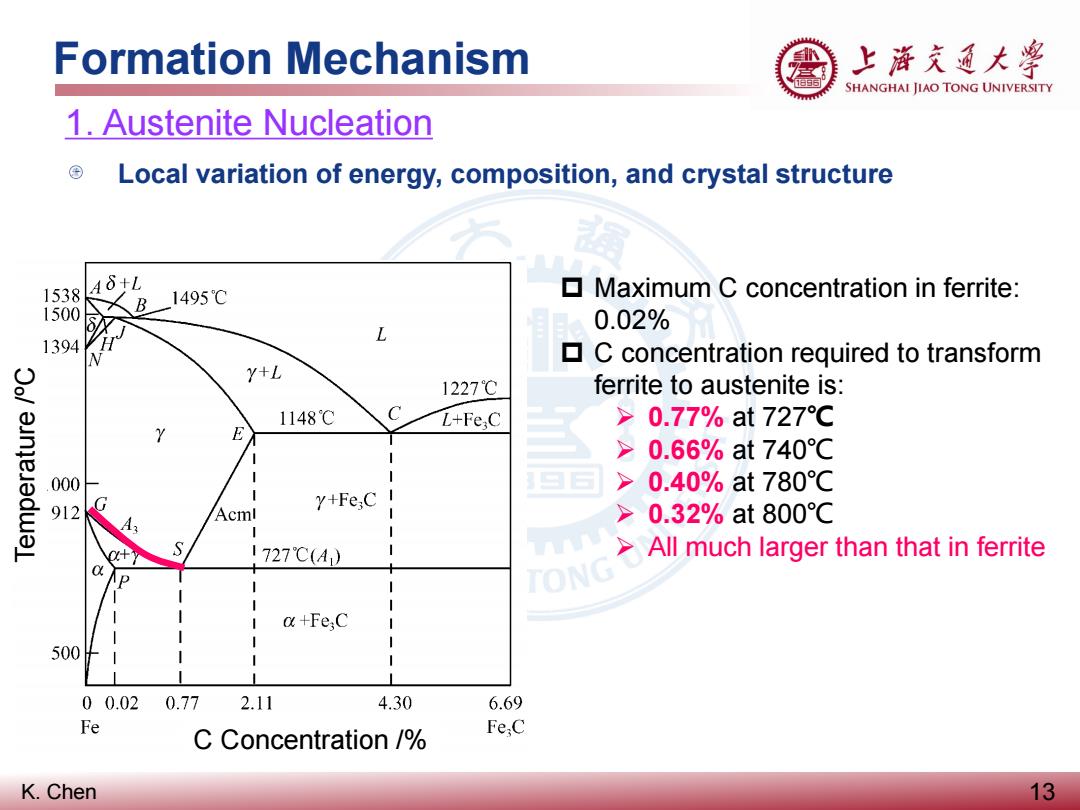
Formation Mechanism 上游克通大粤 SHANGHAI JIAO TONG UNIVERSITY 1.Austenite Nucleation Local variation of energy,composition,and crystal structure 1538 A8+L 1495℃ Maximum C concentration in ferrite: 1500 0.02% L 1394 C concentration required to transform Y+L 1227℃ ferrite to austenite is: 1148℃ L+Fe;C >0.77%at727℃ Y E >0.66%at740℃ 000 >0.40%at780℃ 912 Y+Fe;C I Acml >0.32%at800℃ 1727℃(A) >All much larger than that in ferrite P I a+Fe;C 500 00.02 0.77 2.11 4.30 6.69 Fe C Concentration / Fe;C K.Chen 13
K. Chen Formation Mechanism 13 1. Austenite Nucleation Local variation of energy, composition, and crystal structure Maximum C concentration in ferrite: 0.02% C concentration required to transform ferrite to austenite is: 0.77% at 727℃ 0.66% at 740℃ 0.40% at 780℃ 0.32% at 800℃ All much larger than that in ferrite C Concentration /% Temperature /ºC
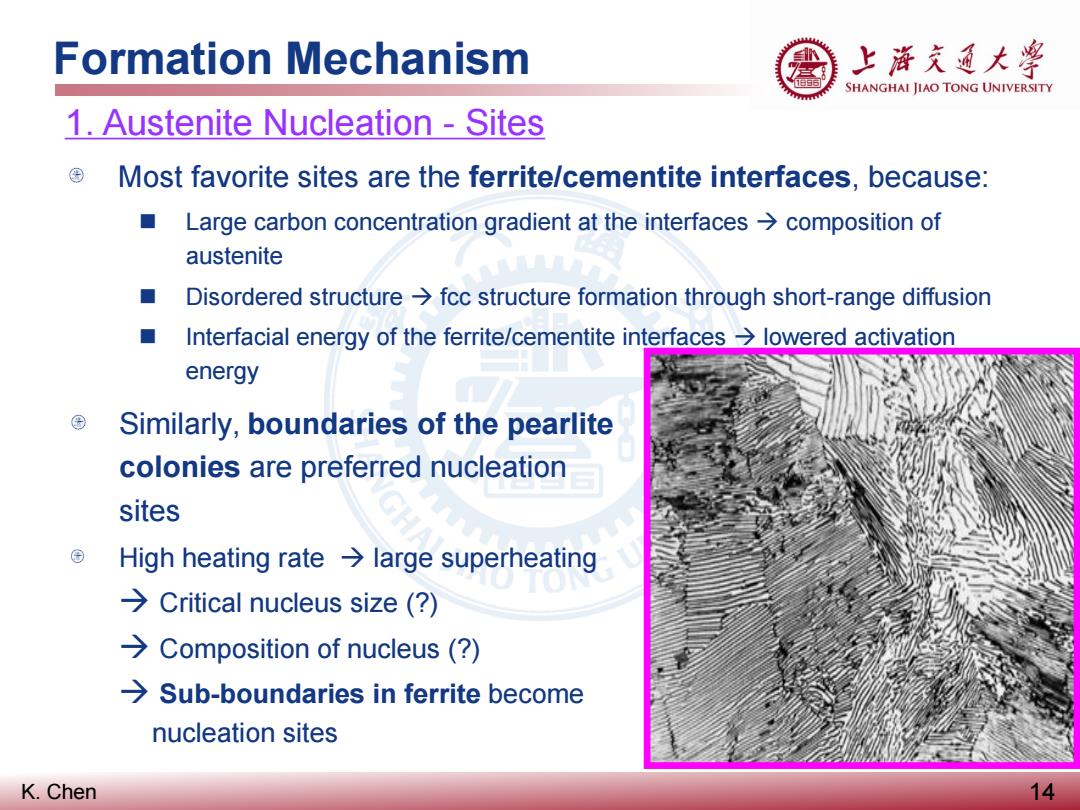
Formation Mechanism 上游充道大粤 SHANGHAI JIAO TONG UNIVERSITY 1.Austenite Nucleation -Sites Most favorite sites are the ferrite/cementite interfaces,because: Large carbon concentration gradient at the interfaces>composition of austenite Disordered structure>fcc structure formation through short-range diffusion ■ Interfacial energy of the ferrite/cementite interfaces lowered activation energy Similarly,boundaries of the pearlite colonies are preferred nucleation sites High heating rate large superheating >Critical nucleus size (? >Composition of nucleus (? >Sub-boundaries in ferrite become nucleation sites K.Chen 14
K. Chen Formation Mechanism 14 1. Austenite Nucleation - Sites Most favorite sites are the ferrite/cementite interfaces, because: Large carbon concentration gradient at the interfaces composition of austenite Disordered structure fcc structure formation through short-range diffusion Interfacial energy of the ferrite/cementite interfaces lowered activation energy Similarly, boundaries of the pearlite colonies are preferred nucleation sites High heating rate large superheating Critical nucleus size (?) Composition of nucleus (?) Sub-boundaries in ferrite become nucleation sites
Formation Mechanism 上游充道大粤 SHANGHAI JIAO TONG UNIVERSITY 2.Austenite Nucleus Growth ⊕ After austenite nucleation,two interphase interfaces formed:y/a and y/Fe C The growth of austenite nucleus is the migration of y/a into a and vWfe3 C into FegC∠目ea Assume all interfaces are planar,carbon concentration in each phase can be determined from the phase diagram of Fe-FeaC AN I JIAO TONG UNIV K.Chen 15
K. Chen Formation Mechanism 15 2. Austenite Nucleus Growth After austenite nucleation, two interphase interfaces formed: γ/α and γ/Fe3C The growth of austenite nucleus is the migration of γ/α into α and γ/Fe3C into Fe3C Assume all interfaces are planar, carbon concentration in each phase can be determined from the phase diagram of Fe-Fe3C