9.2.1 The essence and characteristics of covalent bond Chemical bonding: A chemical bonding is an interaction between atoms or molecules and allows the formation of polyatomic chemical compounds. The theory of chemical bonding consists of 1)The ionic bond theory, 2)The metallic bond theory 3)The covalent bond theory
1)The ionic bond theory, 9.2.1 The essence and characteristics of covalent bond Chemical bonding: A chemical bonding is an interaction between atoms or molecules and allows the formation of polyatomic chemical compounds. The theory of chemical bonding consists of : 2) The metallic bond theory 3) The covalent bond theory

The covalent bond theory 1.The H-H covalent bond formation from isolated Hatoms The two hydrogen atoms of different electronic spin come to each other,overlap resulting in 什H2排斥态 a large overlapped area of electronic probability density. 0 HH基态 The system's energy reduces and the hydrogen molecule is formed. 74 pm R The distance between two nuclei(核间距):R=74pm Essence of covalent bond(共价键的本质)一The atomic orbits overlap to cause increasing electronic probability density which attract the two atomic nuclei
1.The H-H covalent bond formation from isolated H atoms The two hydrogen atoms of different electronic spin come to each other, overlap resulting in a large overlapped area of electronic probability density. The system’s energy reduces and the hydrogen molecule is formed. The distance between two nuclei (核间距): R0 = 74 pm Essence of covalent bond(共价键的本质)——The atomic orbits overlap to cause increasing electronic probability density which attract the two atomic nuclei. The covalent bond theory
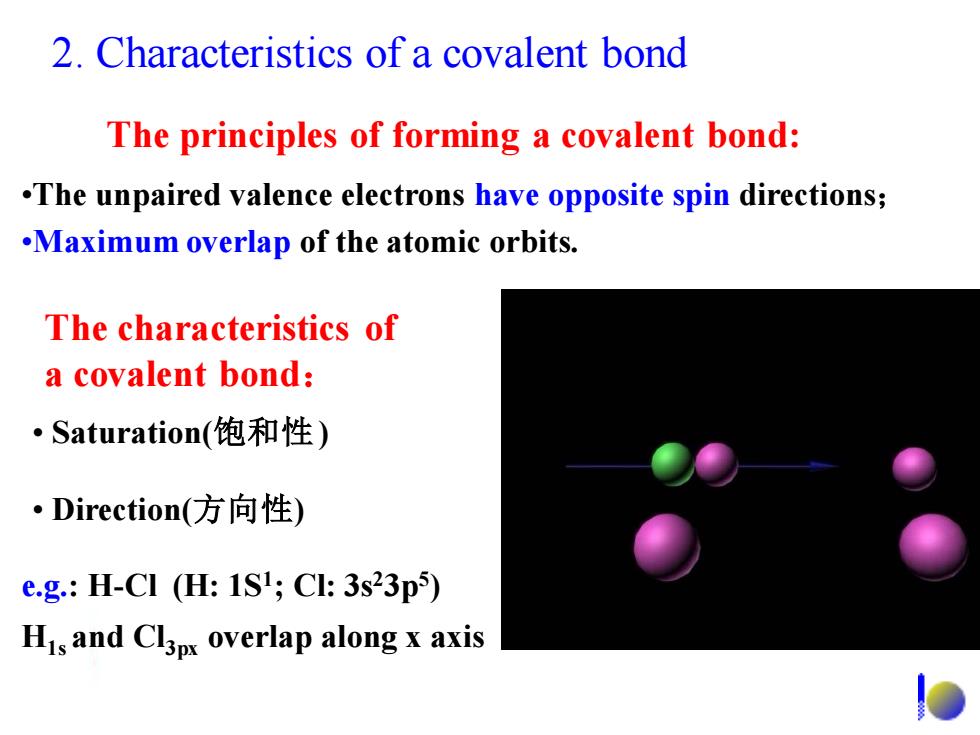
2.Characteristics of a covalent bond The principles of forming a covalent bond: .The unpaired valence electrons have opposite spin directions; .Maximum overlap of the atomic orbits. The characteristics of a covalent bond: Saturation(饱和性) ·Direction(方向性) e.g.:H-CI (H:1SI;Cl:3s23p5) His and Cl3px overlap along x axis
2. Characteristics of a covalent bond The principles of forming a covalent bond: •The unpaired valence electrons have opposite spin directions; •Maximum overlap of the atomic orbits. The characteristics of a covalent bond: • Direction(方向性) • Saturation(饱和性) e.g.: H-Cl (H: 1S1 ; Cl: 3s23p5 ) H1s and Cl3px overlap along x axis
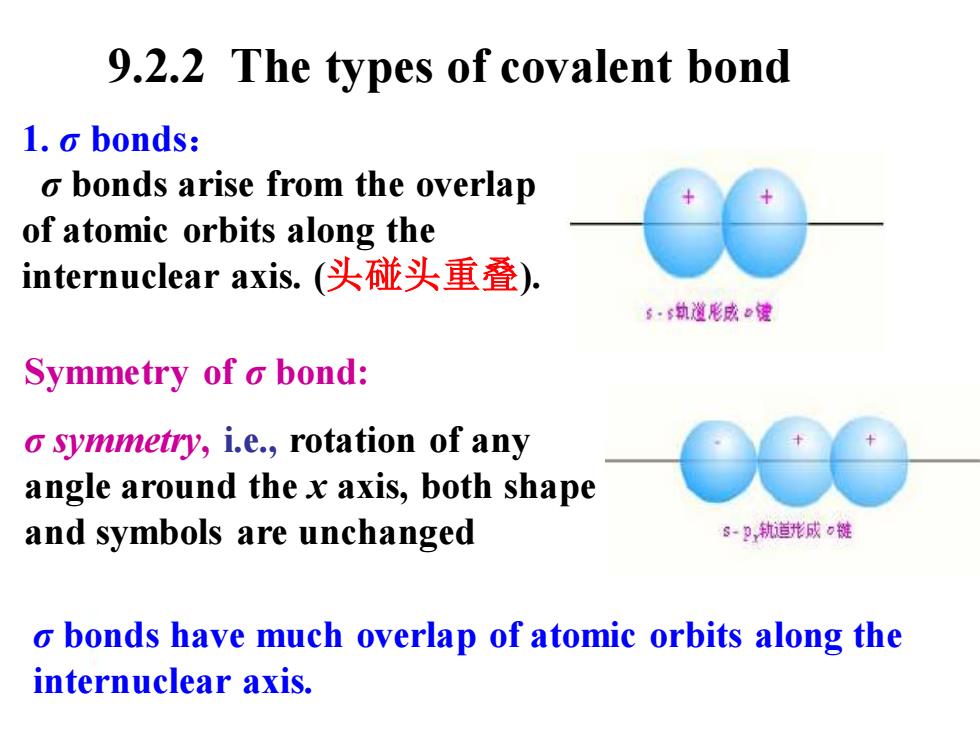
9.2.2 The types of covalent bond 1.o bonds: o bonds arise from the overlap of atomic orbits along the internuclear axis..(头碰头重叠). s·6执道形成e健 Symmetry of o bond: o symmetry,i.e.,rotation of any angle around the x axis,both shape and symbols are unchanged s-P轨道形成0链 o bonds have much overlap of atomic orbits along the internuclear axis
1. σ bonds: σ bonds arise from the overlap of atomic orbits along the internuclear axis. (头碰头重叠). Symmetry of σ bond: σ symmetry, i.e., rotation of any angle around the x axis, both shape and symbols are unchanged σ bonds have much overlap of atomic orbits along the internuclear axis. 9.2.2 The types of covalent bond

S-S s-Px Px-Px o bonds have much overlap of atomic orbits along the internuclear axis
σ bonds have much overlap of atomic orbits along the internuclear axis. s−s px s − px − px