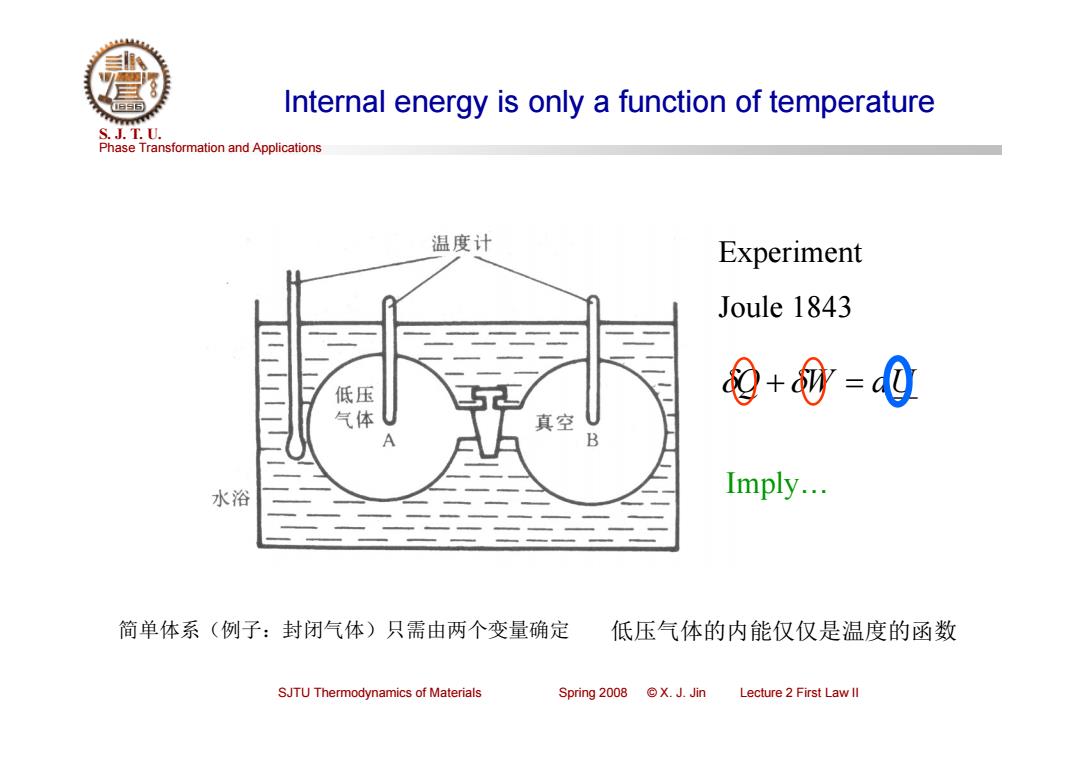
Internal energy is only a function of temperature S.J.T.0. Phase Transformation and Applications 温度计 Experiment Joule 1843 低压 9+0-0 气体 真空 水浴 Imply... 简单体系(例子:封闭气体)只需由两个变量确定 低压气体的内能仅仅是温度的函数 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II Internal energy is only a function of temperature Experiment Joule 1843 Q W dU 简单体系(例子:封闭气体)只需由两个变量确定 低压气体的内能仅仅是温度的函数 Imply…

1.15 Equations of State(2) S.J.T.0. Phase Transformation and Applications Ideal gas its internal energy does not change with volume at constant temperature. H=U+PV =0 ap ap +V =? 〔〔+ aH =0-V+V=0 OH ap 3H =0 av aP SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II 1.15 Equations of State (2) Ideal gas : its internal energy does not change with volume at constant temperature. 0 V T U ? P T H V P V P P U P H T T T H U PV V P RT P P V V U P H T T T 2 0 0 V V PH T ? V T H 0 P T H

1.15 Equations of State(3) S.J.T.0. Phase Transformation and Applications Joule-Thomson coefficient for an ideal gas aP = 工=0 =0 H Euler 02 ay SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II 1.15 Equations of State (3) Joule-Thomson coefficient for an ideal gas. 0 P T H 0 PT H JT THPH PT x y z y z x z x y Euler
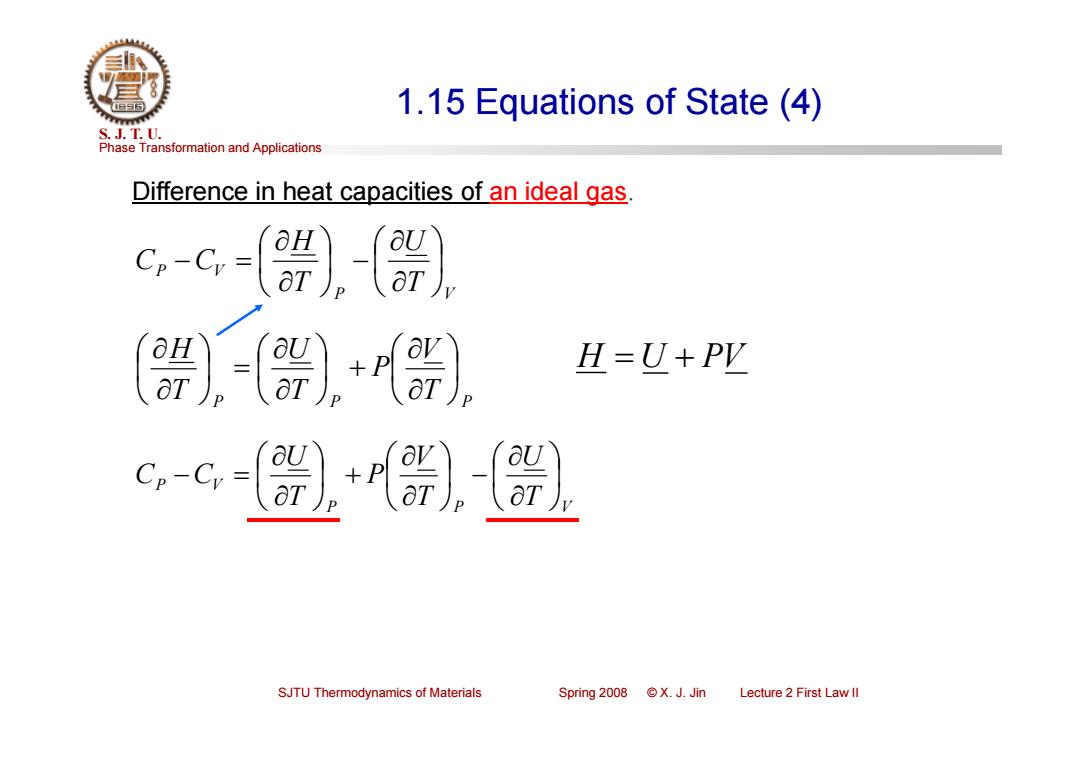
1.15 Equations of State (4) S.J.T.0. Phase Transformation and Applications Difference in heat capacities of an ideal gas 〔-〔器+) H=U+PY c,-G-[2器 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II 1.15 Equations of State (4) Difference in heat capacities of an ideal gas. P V P V T U T H C C H U PV P P T P V P T U T H P P V P V T U T V P T U C C