
reabsorbed by the DCT epithelial cell via an apical Ca2 channel and basolateral Na'/Ca2+exchanger(Figure 4).This process is regulated by parathyroid hormone. Distal convoluted Lumen- tubule Interstitium- urine blood NCC Na Na ATP Ca2+ Ca Na Ca2 Figure 4.Ion transport pathways across the luminal and basolateral membranes of the distal convoluted tubule cell.As in all tubular cells,Na"/K ATPase is present in the basolateral membrane.NCC is the primary sodium and chloride transporter in the luminal membrane.(R,parathyroid hormone [PTH]receptor.) COLLECTING TUBULE The collecting tubule(CCT)is responsible for only 2-5%of NaCl reabsorption by the kidney.Despite this small contribution,the CCT plays an important role in renal physiology and in diuretic action.As the final site of NaCl reabsorption,the collecting tubule is responsible for tight regulation of body fluid volume and for determining the final Na"concentration of the urine.Furthermore,the collecting tubule is a site at which mineralocorticoids exert a significant influence.Lastly,the collecting tubule is the most important site of K*secretion by the kidney and the site at which virtually all diuretic-induced changes in K*balance occur. The mechanism of NaCl reabsorption in the CCT is distinct from the mechanisms found in other tubule segments.The principal cells are the major sites of Na',K, 6
6 reabsorbed by the DCT epithelial cell via an apical Ca2+ channel and basolateral Na+ /Ca2+ exchanger (Figure 4). This process is regulated by parathyroid hormone. Figure 4. Ion transport pathways across the luminal and basolateral membranes of the distal convoluted tubule cell. As in all tubular cells, Na+ /K+ ATPase is present in the basolateral membrane. NCC is the primary sodium and chloride transporter in the luminal membrane. (R, parathyroid hormone [PTH] receptor.) COLLECTING TUBULE The collecting tubule (CCT) is responsible for only 2-5% of NaCl reabsorption by the kidney. Despite this small contribution, the CCT plays an important role in renal physiology and in diuretic action. As the final site of NaCl reabsorption, the collecting tubule is responsible for tight regulation of body fluid volume and for determining the final Na+ concentration of the urine. Furthermore, the collecting tubule is a site at which mineralocorticoids exert a significant influence. Lastly, the collecting tubule is the most important site of K+ secretion by the kidney and the site at which virtually all diuretic-induced changes in K+ balance occur. The mechanism of NaCl reabsorption in the CCT is distinct from the mechanisms found in other tubule segments. The principal cells are the major sites of Na+ , K+

and water transport(Figure 5),and the intercalated cells are the primary sites of H secretion.Unlike cells in other nephron segments,the principal cells do not contain cotransport systems for Na*and other ions in their apical membranes.Principal cell membranes exhibit separate ion channels for Na and K*.Since these channels exclude anions,transport of Na*or K*leads to a net movement of charge across the membrane.Because Na"entry into the principal cell predominates over K"secretion, a 10-50 mV lumen-negative electrical potential develops.Na*that enters the principal cell from the tubular fluid is then transported back to the blood via the basolateral Na/K*ATPase(Figure 5).The 10-50 mV lumen-negative electrical potential drives the transport of Cl back to the blood via the paracellular pathway and draws K out of cells through the apical membrane K channel.Thus,there is an important relationship between Na'delivery to the CCT and the resulting secretion of K" Diuretics that act upstream of the CCT will increase Na'delivery to this site and will enhance K secretion.If the Na'is delivered with an anion that cannot be reabsorbed as readily as Cl(eg,HCO3),the lumen-negative potential is increased,and K" secretion will be enhanced.This mechanism,combined with enhanced aldosterone secretion due to volume depletion,is the basis for most diuretic-induced K'wasting. Reabsorption of Na*via the epithelial Na channel(ENaC)and its coupled secretion of Kis regulated by aldosterone.This steroid hormone,through its actions on gene transcription,increases the activity of both apical membrane channels and the basolateral Na/K*ATPase.This leads to an increase in the transepithelial electrical potential and a dramatic increase in both Na'reabsorption and K*secretion The collecting tubule is also the site at which the final urine concentration is determined.Antidiuretic hormone (ADH,also called arginine vasopressin,AVP) controls the permeability of this segment to water by regulating the insertion of preformed water channels (aquaporin-2,AQP2)into the apical membrane via a G protein-coupled cAMP-mediated process (Figure 6).In the absence of ADH,the collecting tubule (and duct)is impermeable to water and dilute urine is produced. ADH markedly increases water permeability and this leads to the formation of a more concentrated final urine.ADH also stimulates the insertion of urea transporter UT1 molecules into the apical membranes of medullary collecting tubule cells.Urea concentration in the medulla plays an important role maintaining the high osmolarity of the medulla and in the concentration of urine.ADH secretion is regulated by serum osmolality and by volume status. 1
7 and water transport (Figure 5), and the intercalated cells are the primary sites of H+ secretion. Unlike cells in other nephron segments, the principal cells do not contain cotransport systems for Na+ and other ions in their apical membranes. Principal cell membranes exhibit separate ion channels for Na+ and K+ . Since these channels exclude anions, transport of Na+ or K+ leads to a net movement of charge across the membrane. Because Na+ entry into the principal cell predominates over K+ secretion, a 10-50 mV lumen-negative electrical potential develops. Na+ that enters the principal cell from the tubular fluid is then transported back to the blood via the basolateral Na+ /K+ ATPase (Figure 5). The 10-50 mV lumen-negative electrical potential drives the transport of Cl- back to the blood via the paracellular pathway and draws K+ out of cells through the apical membrane K+ channel. Thus, there is an important relationship between Na+ delivery to the CCT and the resulting secretion of K+ . Diuretics that act upstream of the CCT will increase Na+ delivery to this site and will enhance K+ secretion. If the Na+ is delivered with an anion that cannot be reabsorbed as readily as Cl- (eg, HCO3 - ), the lumen-negative potential is increased, and K+ secretion will be enhanced. This mechanism, combined with enhanced aldosterone secretion due to volume depletion, is the basis for most diuretic-induced K+ wasting. Reabsorption of Na+ via the epithelial Na channel (ENaC) and its coupled secretion of K+ is regulated by aldosterone. This steroid hormone, through its actions on gene transcription, increases the activity of both apical membrane channels and the basolateral Na+ /K+ ATPase. This leads to an increase in the transepithelial electrical potential and a dramatic increase in both Na+ reabsorption and K+ secretion. The collecting tubule is also the site at which the final urine concentration is determined. Antidiuretic hormone (ADH, also called arginine vasopressin, AVP) controls the permeability of this segment to water by regulating the insertion of preformed water channels (aquaporin-2, AQP2) into the apical membrane via a G protein-coupled cAMP-mediated process (Figure 6). In the absence of ADH, the collecting tubule (and duct) is impermeable to water and dilute urine is produced. ADH markedly increases water permeability and this leads to the formation of a more concentrated final urine. ADH also stimulates the insertion of urea transporter UT1 molecules into the apical membranes of medullary collecting tubule cells. Urea concentration in the medulla plays an important role maintaining the high osmolarity of the medulla and in the concentration of urine. ADH secretion is regulated by serum osmolality and by volume status
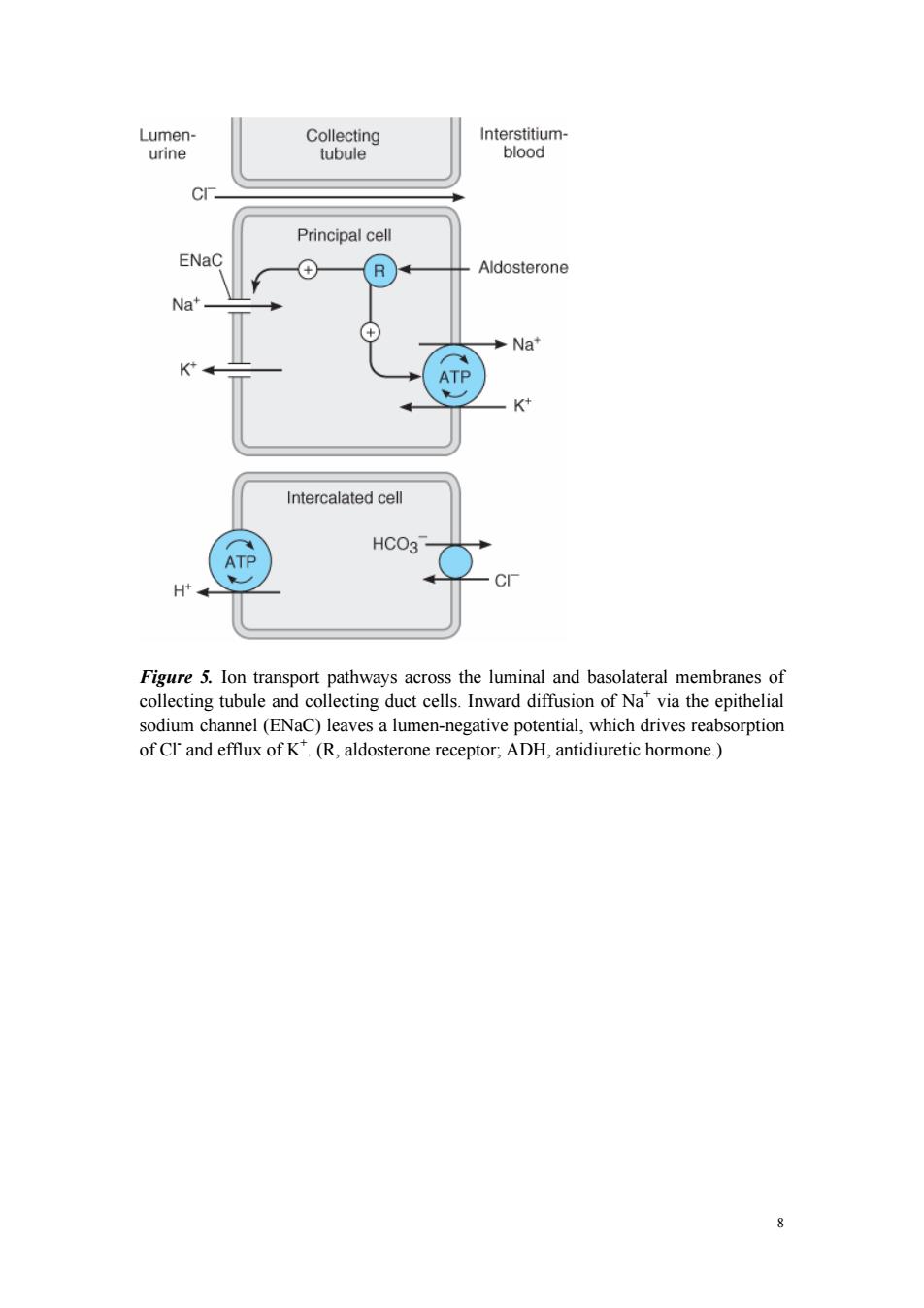
Lumen- Collecting Interstitium- urine tubule blood Principal cell ENaC ⊕ R Aldosterone Na' Na* ATP K Intercalated cell HCO3 ATP H Figure 5.Ion transport pathways across the luminal and basolateral membranes of collecting tubule and collecting duct cells.Inward diffusion of Na"via the epithelial sodium channel(ENaC)leaves a lumen-negative potential,which drives reabsorption of CI'and efflux of K.(R,aldosterone receptor;ADH,antidiuretic hormone.) 8
8 Figure 5. Ion transport pathways across the luminal and basolateral membranes of collecting tubule and collecting duct cells. Inward diffusion of Na+ via the epithelial sodium channel (ENaC) leaves a lumen-negative potential, which drives reabsorption of Cl- and efflux of K+ . (R, aldosterone receptor; ADH, antidiuretic hormone.)
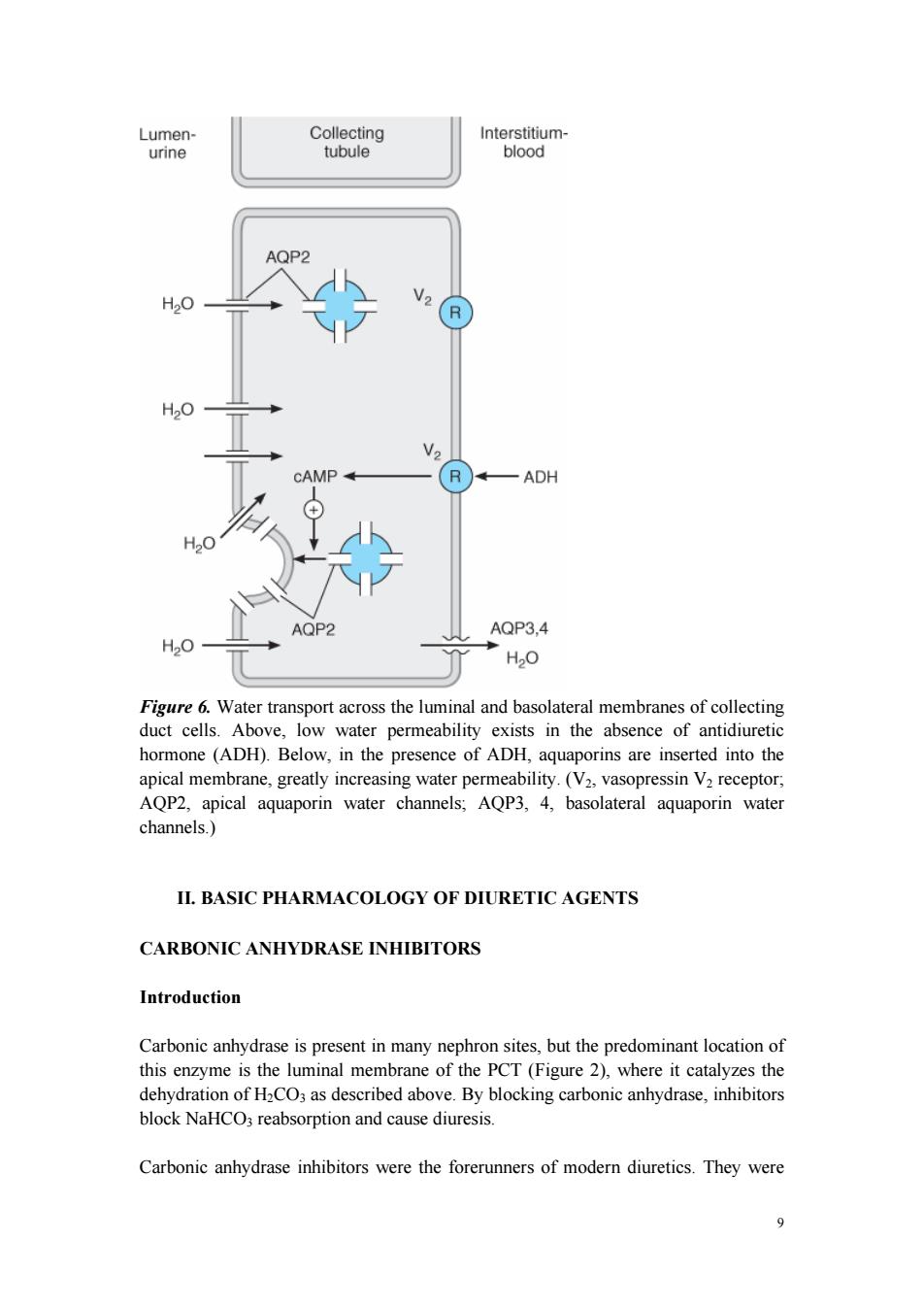
Lumen- Collecting Interstitium- urine tubule blood AQP2 H2O CAMP R —ADH H20 AQP2 AQP3,4 H20 H20 Figure 6.Water transport across the luminal and basolateral membranes of collecting duct cells.Above,low water permeability exists in the absence of antidiuretic hormone(ADH).Below,in the presence of ADH,aquaporins are inserted into the apical membrane,greatly increasing water permeability.(V2,vasopressin V2 receptor, AQP2,apical aquaporin water channels;AQP3,4,basolateral aquaporin water channels.) II.BASIC PHARMACOLOGY OF DIURETIC AGENTS CARBONIC ANHYDRASE INHIBITORS Introduction Carbonic anhydrase is present in many nephron sites,but the predominant location of this enzyme is the luminal membrane of the PCT(Figure 2),where it catalyzes the dehydration of H2CO3 as described above.By blocking carbonic anhydrase,inhibitors block NaHCO3 reabsorption and cause diuresis. Carbonic anhydrase inhibitors were the forerunners of modern diuretics.They were 9
9 Figure 6. Water transport across the luminal and basolateral membranes of collecting duct cells. Above, low water permeability exists in the absence of antidiuretic hormone (ADH). Below, in the presence of ADH, aquaporins are inserted into the apical membrane, greatly increasing water permeability. (V2, vasopressin V2 receptor; AQP2, apical aquaporin water channels; AQP3, 4, basolateral aquaporin water channels.) II. BASIC PHARMACOLOGY OF DIURETIC AGENTS CARBONIC ANHYDRASE INHIBITORS Introduction Carbonic anhydrase is present in many nephron sites, but the predominant location of this enzyme is the luminal membrane of the PCT (Figure 2), where it catalyzes the dehydration of H2CO3 as described above. By blocking carbonic anhydrase, inhibitors block NaHCO3 reabsorption and cause diuresis. Carbonic anhydrase inhibitors were the forerunners of modern diuretics. They were

discovered when it was found that bacteriostatic sulfonamides caused an alkaline diuresis and hyperchloremic metabolic acidosis.With the development of newer agents,carbonic anhydrase inhibitors are now rarely used as diuretics,but they still have several specific applications that are discussed below.The prototypical carbonic anhydrase inhibitor is acetazolamide. Pharmacokinetics The carbonic anhydrase inhibitors are well absorbed after oral administration.An increase in urine pH from the HCO3 diuresis is apparent within 30 minutes,maximal at 2 hours,and persists for 12 hours after a single dose.Excretion of the drug is by secretion in the proximal tubule S2 segment.Therefore,dosing must be reduced in renal insufficiency Pharmacodynamics Inhibition of carbonic anhydrase activity profoundly depresses HCO3 reabsorption in the PCT.At its maximal safely administered dosage,85%of the HCO3 reabsorptive capacity of the superficial PCT is inhibited.Some HCO3'can still be absorbed at other nephron sites by carbonic anhydrase-independent mechanisms,so the overall effect of maximal acetazolamide dosage is only about 45%inhibition of whole kidney HCO3" reabsorption.Nevertheless,carbonic anhydrase inhibition causes significant HCO3" losses and hyperchloremic metabolic acidosis.Because of reduced HCO3 in the glomerular filtrate and the fact that HCO;depletion leads to enhanced NaCl reabsorption by the remainder of the nephron,the diuretic efficacy of acetazolamide decreases significantly with use over several days. At present,the major clinical applications of acetazolamide involve carbonic anhydrase-dependent HCO3 and fluid transport at sites other than the kidney.The ciliary body of the eye secretes HCO3 from the blood into the aqueous humor. Likewise,formation of cerebrospinal fluid by the choroid plexus involves HCO3" secretion.Although these processes remove HCO3 from the blood (the direction opposite to that in the proximal tubule),they are similarly inhibited by carbonic anhydrase inhibitors. Clinical Indications Dosage A.GLAUCOMA The reduction of aqueous humor formation by carbonic anhydrase inhibitors decreases the intraocular pressure.This effect is valuable in the management of glaucoma,making it the most common indication for use of carbonic anhydrase inhibitors.Topically active carbonic anhydrase inhibitors(dorzolamide,brinzolamide) are also available.These topical compounds reduce intraocular pressure,but plasma levels are undetectable.Thus,diuretic and systemic metabolic effects are eliminated 10
10 discovered when it was found that bacteriostatic sulfonamides caused an alkaline diuresis and hyperchloremic metabolic acidosis. With the development of newer agents, carbonic anhydrase inhibitors are now rarely used as diuretics, but they still have several specific applications that are discussed below. The prototypical carbonic anhydrase inhibitor is acetazolamide. Pharmacokinetics The carbonic anhydrase inhibitors are well absorbed after oral administration. An increase in urine pH from the HCO3 - diuresis is apparent within 30 minutes, maximal at 2 hours, and persists for 12 hours after a single dose. Excretion of the drug is by secretion in the proximal tubule S2 segment. Therefore, dosing must be reduced in renal insufficiency. Pharmacodynamics Inhibition of carbonic anhydrase activity profoundly depresses HCO3 - reabsorption in the PCT. At its maximal safely administered dosage, 85% of the HCO3 - reabsorptive capacity of the superficial PCT is inhibited. Some HCO3 - can still be absorbed at other nephron sites by carbonic anhydrase-independent mechanisms, so the overall effect of maximal acetazolamide dosage is only about 45% inhibition of whole kidney HCO3 - reabsorption. Nevertheless, carbonic anhydrase inhibition causes significant HCO3 - losses and hyperchloremic metabolic acidosis. Because of reduced HCO3 - in the glomerular filtrate and the fact that HCO3 - depletion leads to enhanced NaCl reabsorption by the remainder of the nephron, the diuretic efficacy of acetazolamide decreases significantly with use over several days. At present, the major clinical applications of acetazolamide involve carbonic anhydrase-dependent HCO3 - and fluid transport at sites other than the kidney. The ciliary body of the eye secretes HCO3 - from the blood into the aqueous humor. Likewise, formation of cerebrospinal fluid by the choroid plexus involves HCO3 - secretion. Although these processes remove HCO3 - from the blood (the direction opposite to that in the proximal tubule), they are similarly inhibited by carbonic anhydrase inhibitors. Clinical Indications Dosage A. GLAUCOMA The reduction of aqueous humor formation by carbonic anhydrase inhibitors decreases the intraocular pressure. This effect is valuable in the management of glaucoma, making it the most common indication for use of carbonic anhydrase inhibitors. Topically active carbonic anhydrase inhibitors (dorzolamide, brinzolamide) are also available. These topical compounds reduce intraocular pressure, but plasma levels are undetectable. Thus, diuretic and systemic metabolic effects are eliminated