
having various oxidation numbers Outer electron configurations:ns2npl~5 Example:The oxidation numbers of chlorine are+1,+3,+5,+7,-1,0。 Inert electron pair effect(惰性电子对效应): The compounds of ELEMENTS of low oxidation number are more stable than those of high oxidation number when descending in the same groups 同族元素从上到下,低氧化值化合物比高氧化值化合物变得更稳定
Outer electron configurations:ns 2np 1 ~ 5 Example:The oxidation numbers of chlorine are +1,+3,+5,+7,-1,0。 Inert electron pair effect (惰性电子对效应): ◆ having various oxidation numbers The compounds of ELEMENTS of low oxidation number are more stable than those of high oxidation number when descending in the same groups 同族元素从上到下,低氧化值化合物比高氧化值化合物变得更稳定
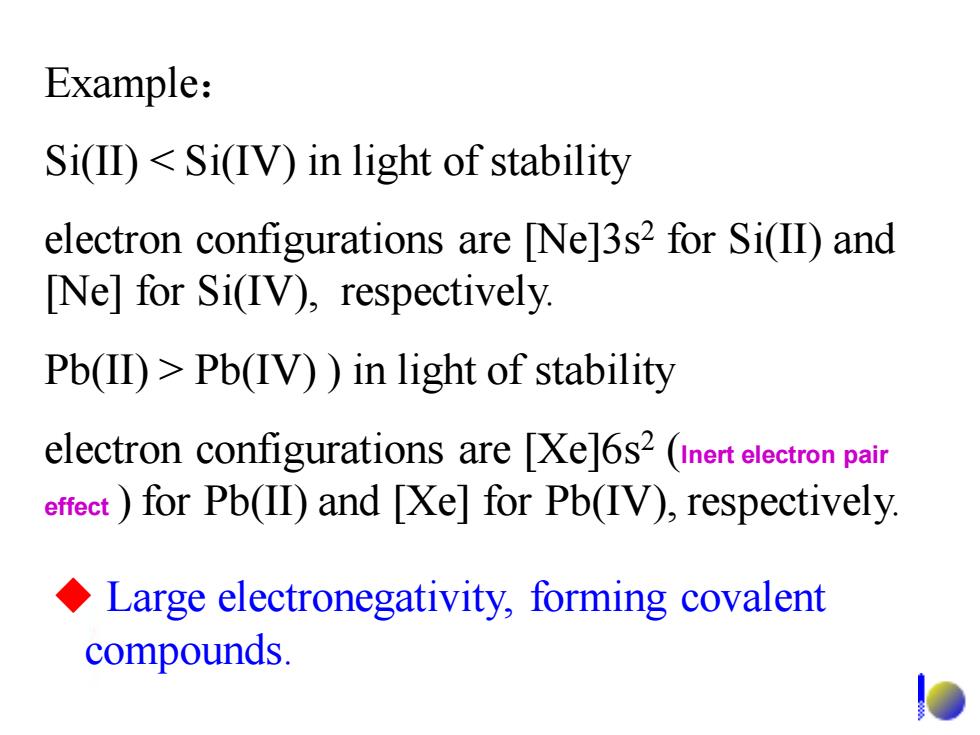
Example: Si(ID)<Si(IV)in light of stability electron configurations are [Ne]3s2 for Si(II)and [Ne]for Si(IV),respectively. Pb(II)>Pb(IV))in light of stability electron configurations are Xe]6s2 (inert electron pair effect for Pb(II)and [Xe]for Pb(IV),respectively Large electronegativity,forming covalent compounds
Example: Si(II) < Si(IV) in light of stability electron configurations are [Ne]3s2 for Si(II) and [Ne] for Si(IV), respectively. Pb(II) > Pb(IV) ) in light of stability electron configurations are [Xe]6s2 (Inert electron pair effect ) for Pb(II) and [Xe] for Pb(IV), respectively. ◆ Large electronegativity, forming covalent compounds

13.2 The elements of boron family 13.2.1 Outline on boron family >13.2.2 The elemental substances of boron family 13.2.3 The compounds of boron -*13.2.4 The compounds of aluminum
§ 13.2 The elements of boron family *13.2.4 The compounds of aluminum 13.2.3 The compounds of boron 13.2.2 The elemental substances of boron family 13.2.1 Outline on boron family

13.2.1 Outline on boron family Boron family (IA):B,Al,Ga,In,TI Valence electron configuration:ns2npl Electron deficient elements:number of valence electrons number of valence orbits Electron deficient compounds:number of electron pairs in bonding molecular orbits number of valence orbits(即:还有空的成键轨道) Example:BF3(sp3),H3BO3.B:2S22pl HBF is not electron deficient compound
13.2.1 Outline on boron family Boron family (ⅢA):B,Al,Ga,In,Tl Valence electron configuration:ns 2np 1 Electron deficient elements: number of valence electrons < number of valence orbits Electron deficient compounds: number of electron pairs in bonding molecular orbits < number of valence orbits(即:还有空的成键轨道). Example:BF3 (sp3 ), H3BO3。 B: 2S22p1 HBF4 is not electron deficient compound

2p 2P excited 2s $p2 B:2s22p1 sp2 hybridization The formation of BF
of BF3 The formation 2s 2p 2p 2s sp 2 sp2 hybridization B : 2s 22p 1 excited