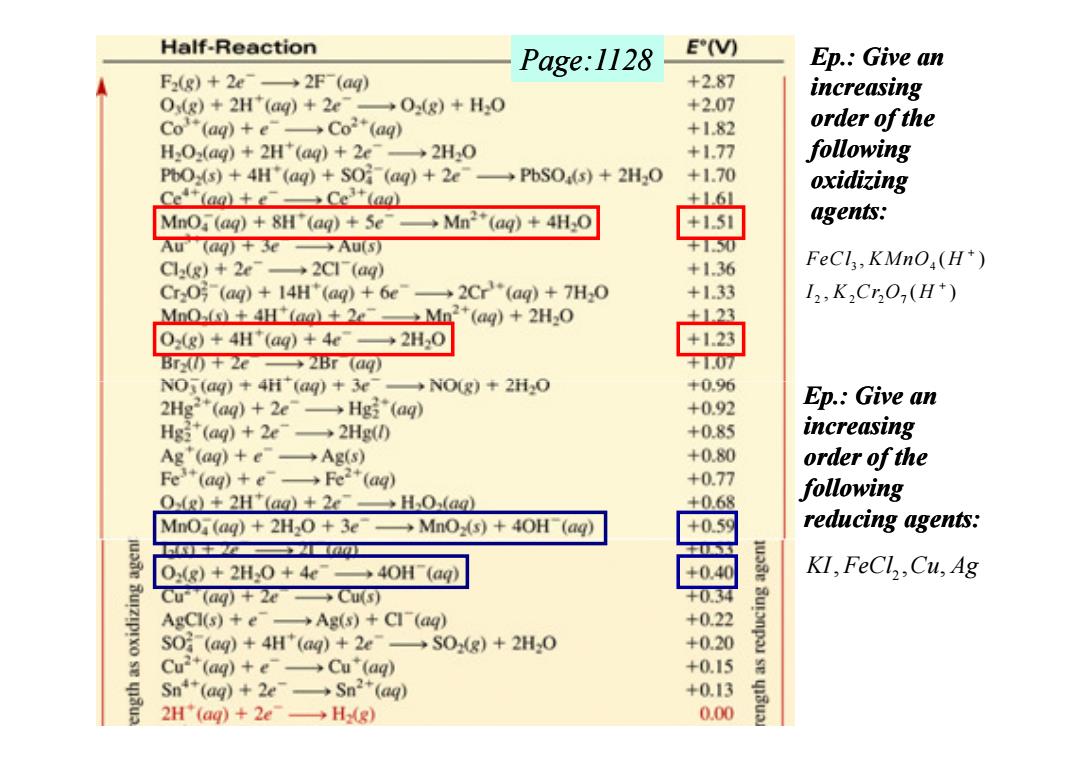
Half-Reaction Page:1128 E(V) Ep.:Give an F(g)+2e→2F(ag) +2.87 increasing Ox(g)+2H"(aq)+2e-02(g)+H2O +2.07 Co“(ag)+e→Co2*(ag +1.82 order of the H2Oz(ag)+2H*(ag)+2e-2H2O +177 following PbOx(s)+4H"(ag)+SO(ag)+2e-PbSO(s)+2H2O +1.70 oxidizing Cet(aa)+eCe(ao) MnO (aq)8H"(aq)+5e →Mn2(ag)+4Hs0 agents: AU(@g+3e→AuS F1.0 C(g)+2e→2C1(aq) +136 FeCl,KMnO(H*) Cr2-(ag)14H*(ag)+6e--2Cr(aq)+7H2O +1.33 I2,K2C5O,(H+) Mno-(s)+4H(ag+2eMn2*(aq)+2H20 +123 0(g)+4H(aq)+4e→2H,0 +1.23 BT+2e→2Br(a网 NOj(aq)+4H(aq)3e-NO(g)+2H2O +0.96 2Hg2*(aq)+2e→Hgi(aq) +0.92 Ep.:Give an Hg3(aq)+2e→2Hg0 +0.85 increasing Ag'(ag)+e→Ag(s) +0.80 order of the Fe3+(ag)+e→Fe2+(ag) +0.77 O(g)+2H"(ag)+2eHO(ag) +0.68 following MnO (ag)+2H2O +3e-MnOz(s)+40H (ag) +0.59 reducing agents: 4■ 0(g)+2H,0+4e→40H(ag) +0.40 KI,FeCl,,Cu,Ag Cu(aq)+2e→Cu(S) AgCl(s)+e-Ag(s)+Cl(aq) +0.22 S0(aq)+4H'(aq)+2e→S0(g)+2H,0 +0.20 Cu2*(ag)+e→Cu(ag) +0.15 sn(ag)+2e→Sn2+(ag) +0.13 2H'(ag)+2e→HR) 0.00
3 4 2 2 2 7 , ( ) , ( ) FeCl KMnO H I K Cr O H + + Ep.: Give an increasing order of the following oxidizing agents: Ep.: Give an Page:1128 2 KI FeCl Cu Ag , , , Ep.: Give an increasing order of the following reducing agents:

Standard reduction Potentials at 25C 2H(ag)+2e→Hg) 0.00 Pb2*(aq)+2e →Pb(s) -0.13 The better oxidizer sn2*(aq)+2e→Sn -0.14 Nr2*(aq)+2e→Ni() CoCL2 or Cr(NO)3 -0.25 Co2*(aq)+2e →Cs) -0.28 PbSO (s)+2e →Pbs)+s0i(ag) -031 Cd+(aq)+2e→Cds) -0.40 Fe2(aq)+2c→Fe) -0.44 Cr3(ag)+3e→Cr -0.74 Zn2ag)+2e→Zn9 -076 2H,0+2e→Hg)+20H(ag) -0.83 Mn2t(ag)+2e→Mns) -1.18 AI3*(aq)+3e→Als) -1.66 Be2(aq)+2e→Bc9 -1.85 Mg2+(aq)+2e→Mg9 -237 Na'(aq)+e→Nas) -2.71 The better reducer Ca2(ag)+2e→Cas) -2.87 Ni(s)or Zn(母 sr2+(aq)+2e→Srt9 -2.89 Ba2(aq)+2e→Bas) -2.90 K(ag)+e→K(s) -293 Li(aq)+e→Li(s) -3.05
Standard reduction Potentials at 25ºC The better oxidizer ? CoCl2 or Cr(NO 3 ) 3 The better reducer ? Ni(s) or Zn(s)
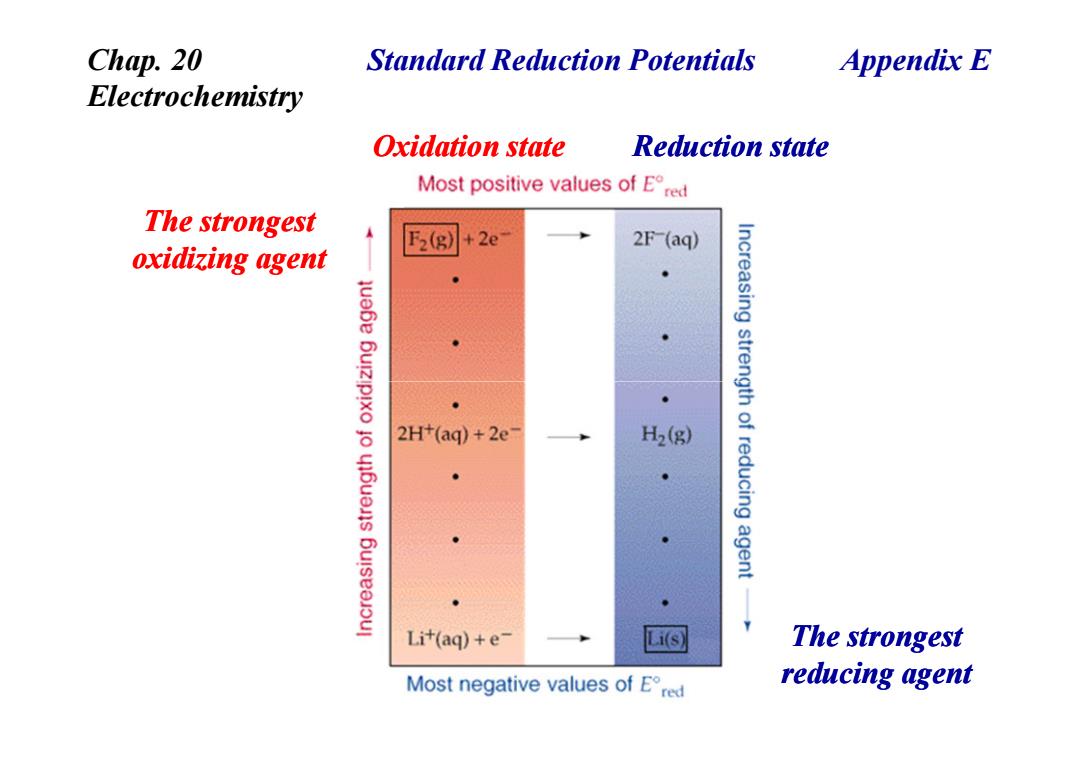
Chap.20 Standard Reduction Potentials Appendix E Electrochemistry Oxidation state Reduction state Most positive values of Ered The strongest F2(g) 2e 2F (aq) oxidizing agent 2H+(aq)+2e H2(g) Increasing strength of reducing agent Lit(aq)+e- Li(s) The strongest Most negative values of Ered reducing agent
Standard Reduction Potentials Oxidation state Reduction state Chap. 20 Electrochemistry The strongest oxidizing agent Appendix E The strongest reducing agent
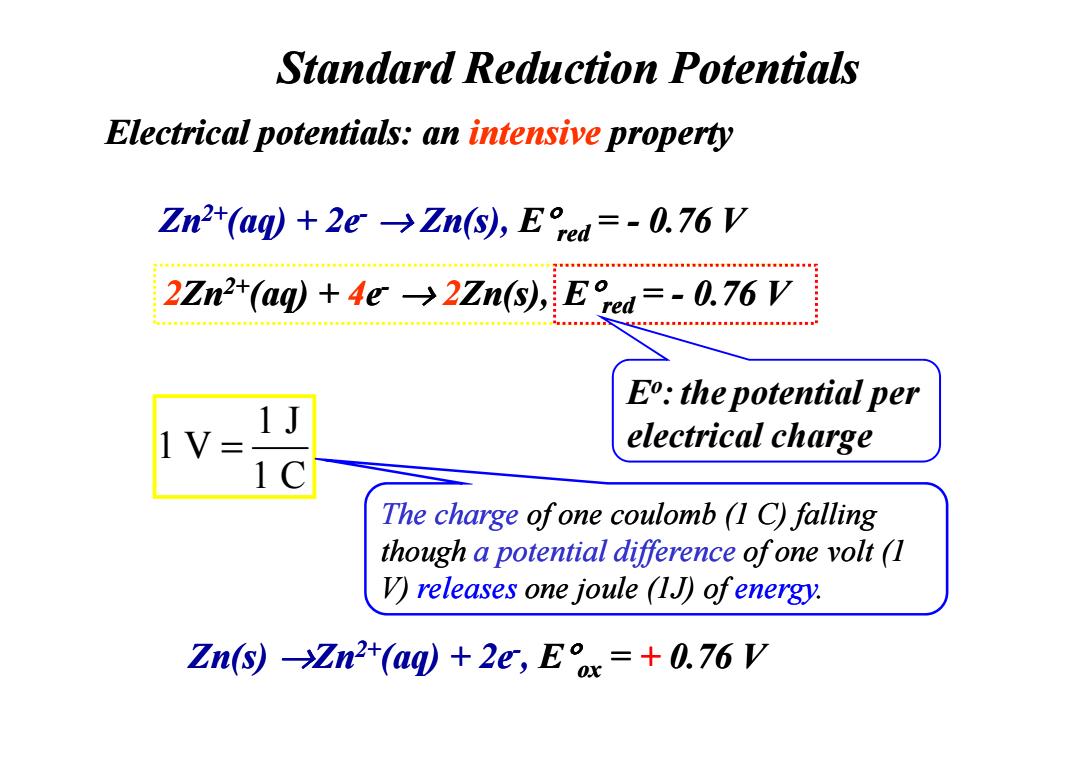
Standard Reduction Potentials Electrical potentials:an intensive property Zn2+(aq)+2e->Zn(s),Eed=-0.76 V 2Zn2+(aq)+4e>2Zn(s),E red=-0.76 V E:the potential per V electrical charge The charge ofone coulomb (1 C)falling though a potential difference of one volt(I V)releases one joule (1J)ofenergy. Zn(s)Zn2t(aq)+2e,Ex=+0.76 V
Zn2+(aq) + 2e- → Zn(s), E°red = - 0.76 V Standard Reduction Potentials Electrical potentials: an intensive property 2Zn2+(aq) + 4e- → 2Zn(s), E°E°red red= ? V = - 0.76 V Eo:the potential per electrical charge Zn(s) →Zn2+(aq) + 2e-, E°ox = + 0.76 V 1 J 1 V 1 C = The charge of one coulomb (1 C) falling though a potential difference of one volt (1 V) releases one joule (1J) of energy

Spontaneity of Redox Reactions 自发性 氧化还原 △G:the maximum amount of energy for useful work △H-T△S=△G=W non-exp ansion △H-T△S=△G=Welecrical work △G=Welecrical work Gibbs free energy Cell potential
Spontaneity of Redox Reactions ∆ − ∆ = ∆ = H T S G Wnon ansion -exp ∆G : the maximum amount of energy for useful work ∆ − ∆ = ∆ = H T S G Welectrical work 自发性 氧化还原 Gibbs free energy electrical work ? ? ∆G E → → W cell ∆ = G Welectrical work ? ? Cell potential