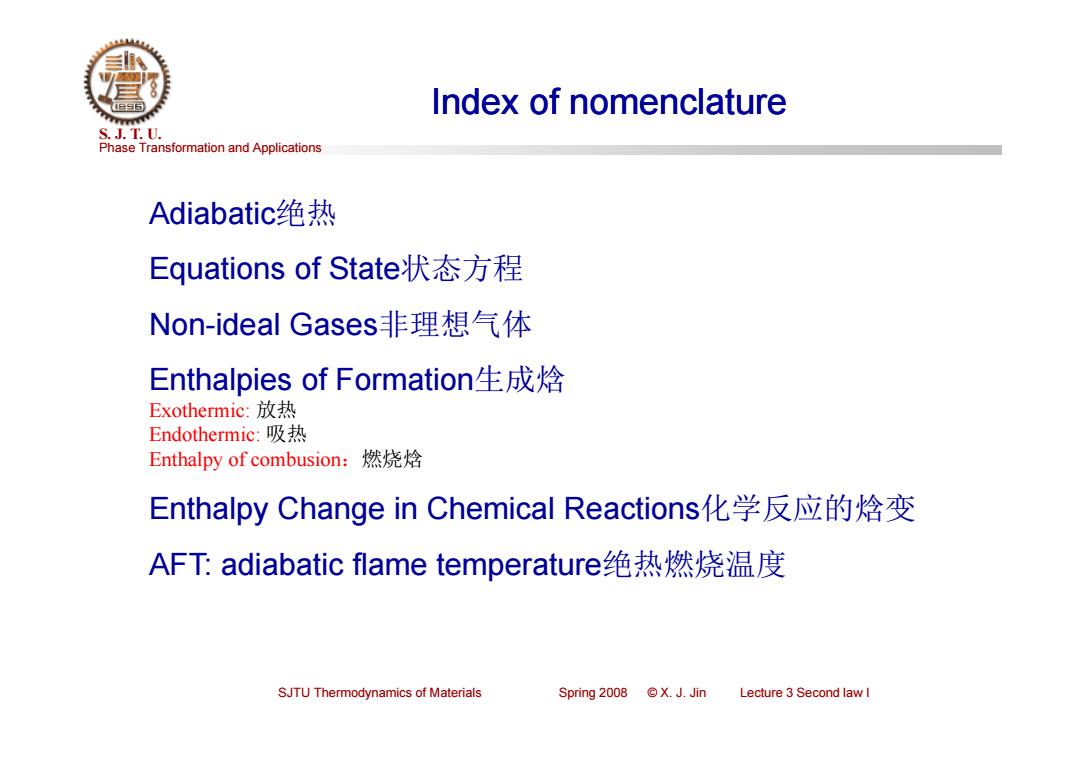
Index of nomenclature S.J.T.0. Phase Transformation and Applications Adiabatic绝热 Equations of State状态方程 Non-ideal Gases非理想气体 Enthalpies of Formation生成焓 Exothermic:放热 Endothermic:吸热 Enthalpy of combusion: 燃烧焓 Enthalpy Change in Chemical Reactions化学反应的焓变 AFT:adiabatic flame temperature绝热燃烧温度 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I Index of nomenclature Adiabatic绝热 Equations of State状态方程 Non-ideal Gases非理想气体 Enthalpies of Formation生成焓 Exothermic: 放热 Endothermic: 吸热 Enthalpy of combusion:燃烧焓 Enthalpy Change in Chemical Reactions化学反应的焓变 AFT: adiabatic flame temperature绝热燃烧温度 Index of nomenclature
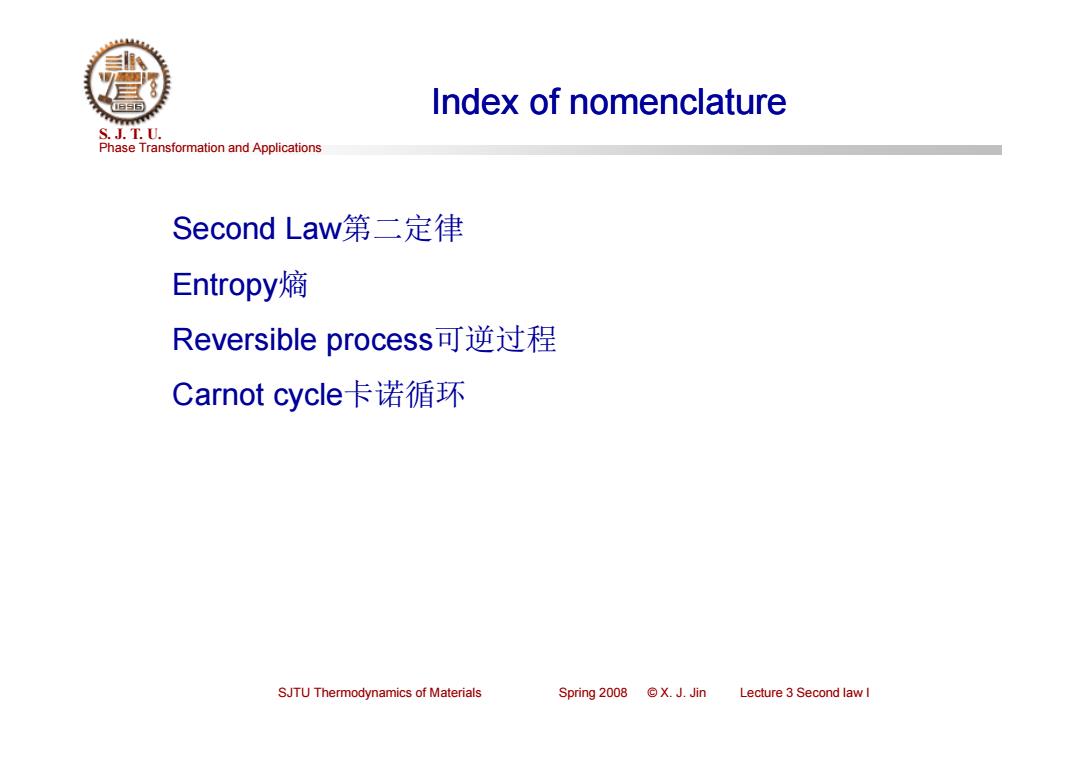
Index of nomenclature S.J.T.0. Phase Transformation and Applications Second Law第二定律 Entropy熵 Reversible process可逆过程 Carnot cycle卡诺循环 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I Index of nomenclature Second Law第二定律 Entropy熵 Reversible process可逆过程 Carnot cycle卡诺循环 Index of nomenclature

The Second Law S.J.T.0. Phase Transformation and Applications The First Law:the conservation of energy and energy transfer in terms of heat and work. Energy is a state function. The Second Law:ENTROPY. Overview Heat engines,devices that convert thermal energy (heat)into mechanical energy (work). The first law places no limits on the amounts that can be converted. The second law is concerned with limits on the conversion of"heat"into s“work”by heat engines. 1824,a French engineer,Sadi Carnot,idealized heat engine. P.W.Atkins,The Second Law,Scientific American Books,1984 SJTU Thermodynamics of Materials Spring2o08©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I The Second Law The First Law: the conservation of energy and energy transfer in terms of heat and work. Energy is a state function. The Second Law: ENTROPY. Overview Heat engines, devices that convert thermal energy (heat) into mechanical energy (work). The first law places no limits on the amounts that can be converted. The second law is concerned with limits on the conversion of “heat” into “work” by heat engines. 1824, a French engineer, Sadi Carnot, idealized heat engine. P. W. Atkins, The Second Law, Scientific American Books, 1984
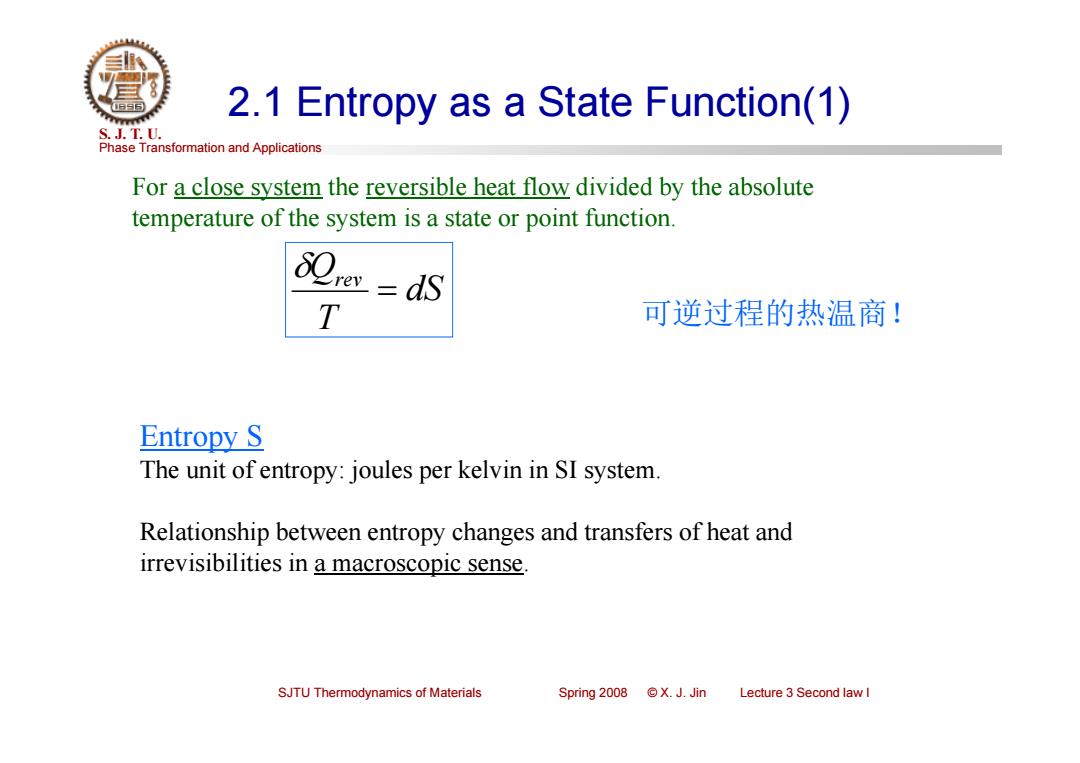
2.1 Entropy as a State Function(1) S.J.T.0. Phase Transformation and Applications For a close system the reversible heat flow divided by the absolute temperature of the system is a state or point function. T 可逆过程的热温商! Entropy S The unit of entropy:joules per kelvin in SI system. Relationship between entropy changes and transfers of heat and irrevisibilities in a macroscopic sense. SJTU Thermodynamics of Materials Spring 2008 ©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I 2.1 Entropy as a State Function(1) For a close system the reversible heat flow divided by the absolute temperature of the system is a state or point function. Entropy S The unit of entropy: joules per kelvin in SI system. Relationship between entropy changes and transfers of heat and irrevisibilities in a macroscopic sense. dS T Qrev 可逆过程的热温商!
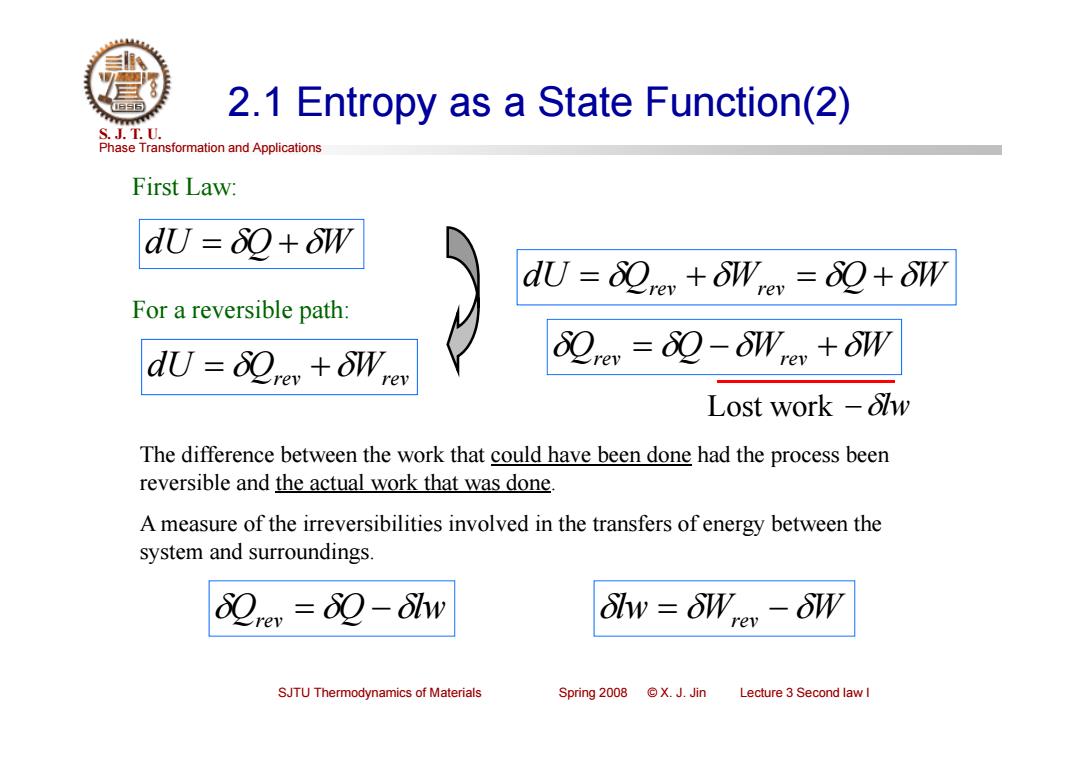
2.1 Entropy as a State Function(2) S.J.T.0. Phase Transformation and Applications First Law: dU=80+oW du=0o+Wron =80+8W For a reversible path: du=sre+oWrer 0re=80-Wey +oW Lost work -olw The difference between the work that could have been done had the process been reversible and the actual work that was done A measure of the irreversibilities involved in the transfers of energy between the system and surroundings. 0rer=80-8tw 8lw=8W,-8W SJTU Thermodynamics of Materials Spring2o08©X.J.Jin Lecture 3 Second law I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I 2.1 Entropy as a State Function(2) First Law: dU Q W dU Qrev Wrev For a reversible path: dU Qrev Wrev Q W Qrev Q Wrev W Lost work lw The difference between the work that could have been done had the process been reversible and the actual work that was done. A measure of the irreversibilities involved in the transfers of energy between the system and surroundings. Qrev Q lw lw Wrev W