The Colors of hydrate-isomers of complexes of Cr(II)ion [Cr(H2O]Cl3 purple,[Cr(H2O)3CI]Cl2.H2Ocyan 蓝绿色 [Cr(H,O)CI,]CI.2H,O green Cr3+:d2sp3 4.The conversion between Cr2O2-and CrO2-ions ·The effect of pH 2CrO+2H-2HCrO-Cr2O+H2O yellow Orrange pH<2:Cr2O2 predominates pH>6:CrO2 predominates
• The Colors of hydrate-isomers of complexes of Cr(Ⅲ) ion [Cr(H O Cl ]Cl 2H O green [Cr(H O ]Cl purple [Cr(H O Cl]Cl H O cyan 2 4 2 2 2 6 3 2 5 2 2 ) ) , ) • The effect of pH pH<2:Cr2O7 2- predominates ; pH>6:CrO4 2- predominates 2CrO 2H 2HCrO Cr O H2O 2 4 2 7 2 4 + + - + - - (黄) (橙) 4.The conversion between Cr2O7 2- and CrO4 2- ions 蓝绿色 Cr3+: d2 sp3 yellow Orrange
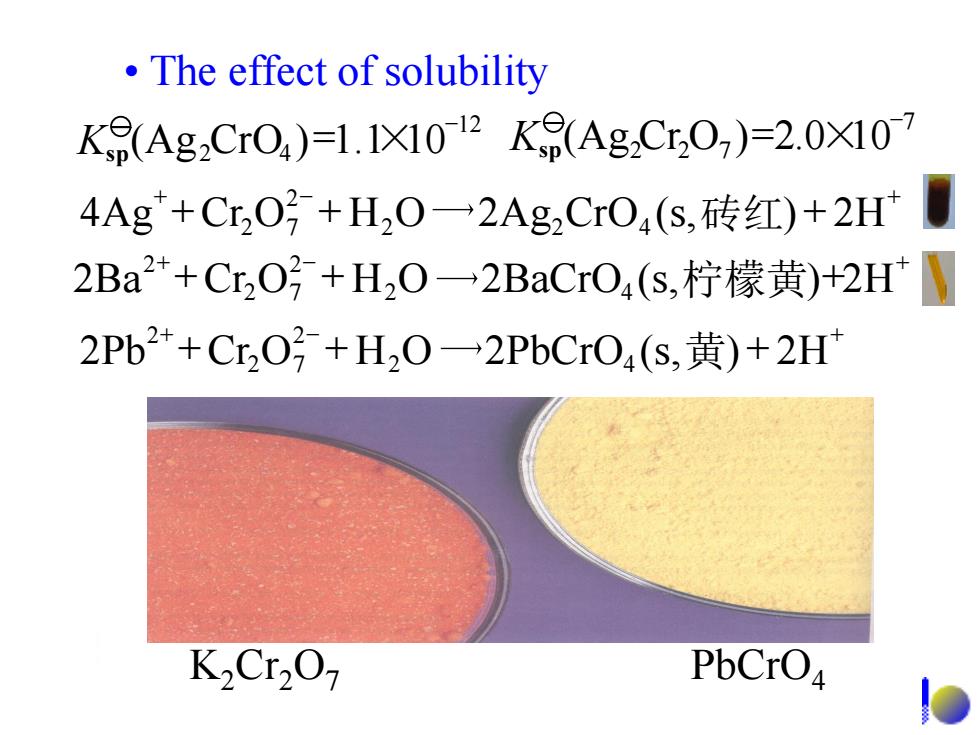
The effect of solubility Kf(Ag2CrO4)=1.1X102Kf(AgCr,07)=2.0X107 4Ag+C,0号+H,0一2Ag,Cr04(s,砖红)+2H 2Ba2+C,O号+H,0一2 BaCrO4(s,柠檬黄)+2HW 2Pb2+Cr,O号+H20一2 PbCrO4(s,黄)+2H K2Cr2O PbCrO4
• The effect of solubility - (Ag Cr O )=2.0×10 7 Ksp 2 2 7 - (Ag CrO )=1.1×10 12 Ksp 2 4 + - + 4Ag +Cr O + H2O 2Ag2CrO4 (s, ) + 2H 2 2 7 砖红 + - + 2Ba +Cr O + H2O 2BaCrO4 (s, )+2H 2 2 7 2 柠檬黄 + - + 2Pb +Cr O + H2O 2PbCrO4 (s, ) + 2H 2 2 7 2 黄 K2Cr2O7 PbCrO4