
21.2 The compounds of chromium CrO3(铬绿) color m.p./℃ changes when heated 暗红色 250℃分解为 CrO3(铬酐) 198 Cr203与02 K,CrO 黄色 975 熔融不分解 K,Cr02(红矾) 橙红色 398 熔融不分解 Cr,O3(铬绿) 绿色 2330 不分解 CrCl 6H2O 紫色 83 失去结晶水 KCr(SO)2 12H2O 暗紫色 89 失去结晶水
color m.p./℃ changes when heated CrO3 (铬酐) 暗红色 198 250℃分解为 Cr2O3与O2 K2CrO4 黄色 975 熔融不分解 K2Cr2O7 (红矾) 橙红色 398 熔融不分解 Cr2O3 (铬绿) 绿色 2330 不分解 CrCl3·6H2O 紫色 83 失去结晶水 KCr(SO4 )2·12H2O 暗紫色 89 失去结晶水 Cr2O3 (铬绿) 21.2 The compounds of chromium

C,02 Different ions of Cr in aqueous solution color pH for their existence 3 Cr,03 橙红 <2 50 CrO CrO2 黄 >6 Cr3+(aq) 紫 酸性 Cr(OH) 亮绿 强碱 Cr2+(aq)Cr3+(aq) Cr2+(aq) 蓝 酸性
color pH for their existence 橙红 <2 黄 >6 Cr3+(aq) 紫 酸性 亮绿 强碱 Cr2+(aq) 蓝 酸性 Different ions of Cr in aqueous solution 2- Cr2 O7 2- CrO4 - Cr(OH)4 Cr2+(aq) Cr3+(aq) 2- Cr2 O7 2- CrO4
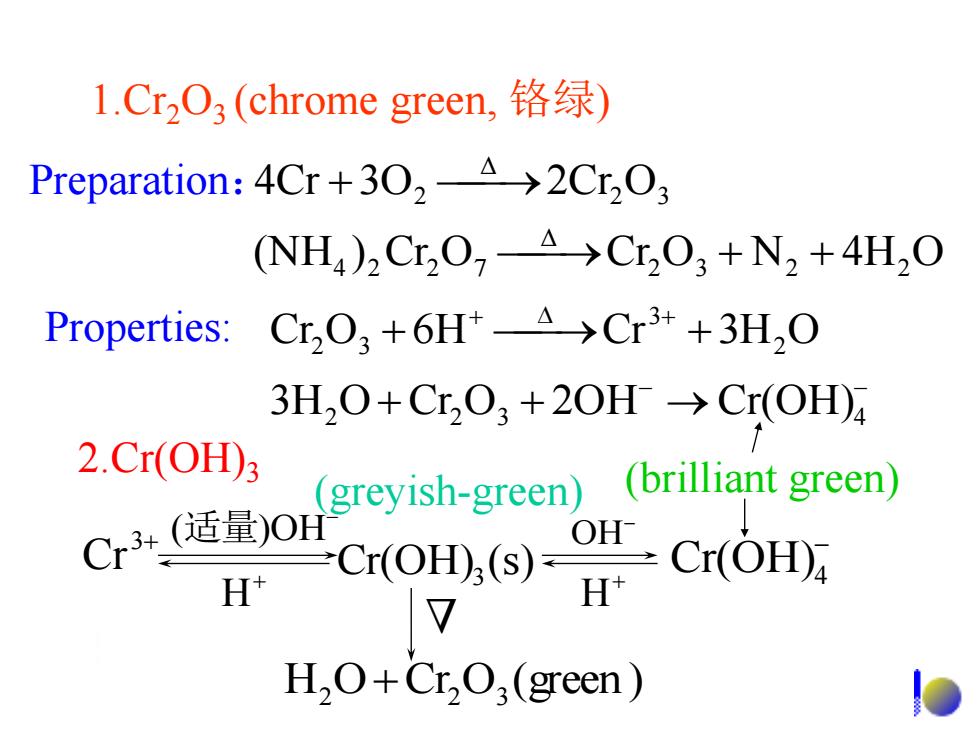
1.CrO3(chrome green,铬绿) Preparation:4Cr+302-A>2Cr2O3 (NH)2Cr2O7-A >Cr2O;+N2+4H2O Properties:Cr,O,+6H*A>Cr3++3H,O 3H2O+Cr2O3 +20H>Cr(OH) 2.Cr(OH)3 Cr3适量到)0H greyish-green)(brilliant green) H rot),) H2O+Cr,O(green)
Preparation: Properties: (NH ) Cr O Cr O N 4H O 4Cr 3O 2Cr O 2 3 2 2 Δ 4 2 2 7 2 3 Δ 2 + + + - - + + + + + + 2 2 3 4 2 3 2 3 3H O Cr O 2OH Cr(OH) Cr O 6H Cr 3H O 3+ Cr - Cr(OH)4 Cr(OH) (s) 3 H O Cr O (green ) 2 + 2 3 - (适量)OH - OH + H (greyish-green) (brilliant green) Δ 1.Cr2O3 (chrome green, 铬绿) 2.Cr(OH)3 + H
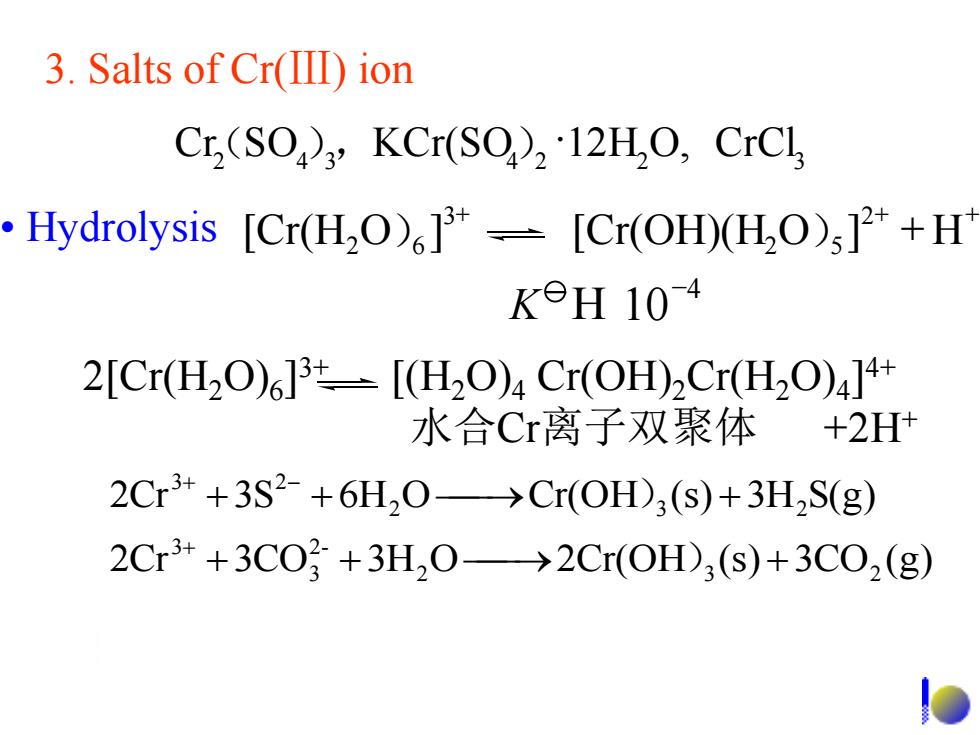
3.Salts of Cr(IID)ion Cr,(SO3,KCr(SO),12HO,CrCl Hydrolysis [Cr(H2O)]-[Cr(OH)(HO)3]+H K9H104 2[Cr(H2O)6]3t=[(H2O)4Cr(OH)2Cr(H2O)4]4+ 水合Cr离子双聚体 +2H+ 2Cr3++3S2-+6H,0→Cr(OH)3(S)+3H2S(g) 2Cr3++3C03+3H20→2Cr(OH),(s)+3C02(g)
2 4 3 4 2 2O, CrCl3 Cr(SO),KCr(SO)12H 2Cr 3CO 3H O 2Cr(OH (s) 3CO (g) 2Cr 3S 6H O Cr(OH (s) 3H S(g) 2 3 2 2- 3 3 2 3 2 3 2 + + + + + + + + - ) ) 3. Salts of Cr(Ⅲ) ion • Hydrolysis + + + [Cr(H O ] [Cr(OH)(H O ] + H 2 2 5 3 2 )6 ) 4 10- K 2[Cr(H2O)6 ] 3+ [(H2O)4 Cr(OH)2Cr(H2O)4 ] 4+ 水合Cr离子双聚体 +2H+
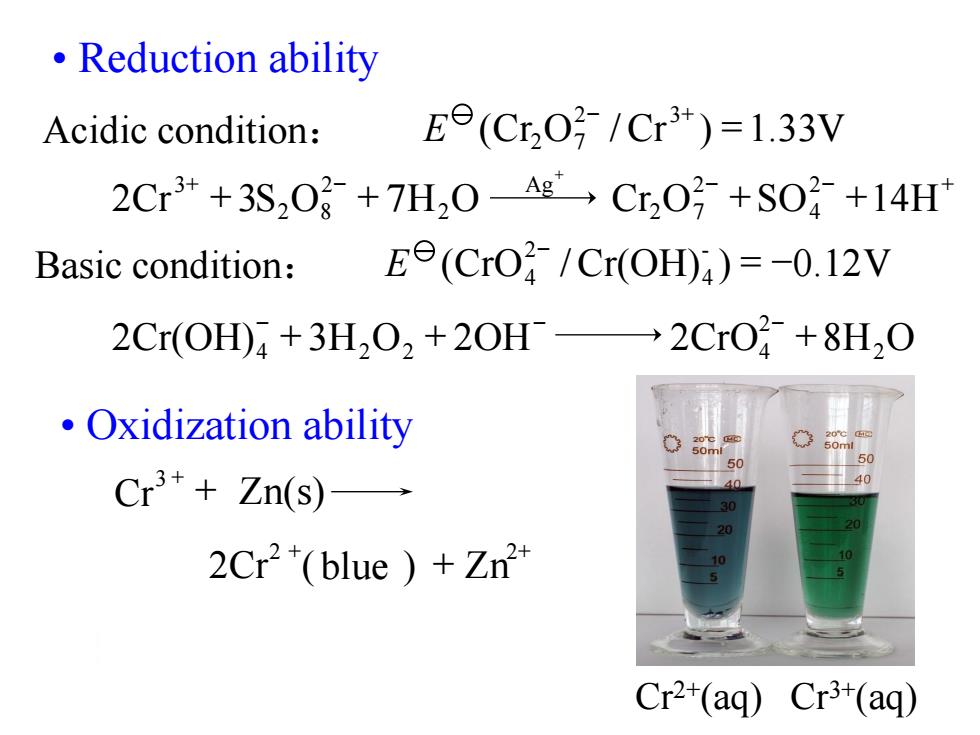
·Reduction ability Acidic condition: Ee(Cr,03/Cr3*)=1.33V 2Cr*+3S2O+7H2O-Ag Cr2O+SO+14H Basic condition: E(CrO/Cr(OH))=-0.12V 2Cr(OH)+3H2O2+20H-2CrO+8H,O Oxidization ability Cr3++Zn(s)- 2Cr"(blue Zn2 Cr2+(aq)Cr3+(aq)
• Reduction ability • Oxidization ability 2Cr ( blue ) Zn 2 + 2+ + Cr Zn(s) 3 + + 2Cr(OH) 3H O 2OH 2CrO 8H2O 2 4 2 2 4 + + + - - - 2Cr 3S O 7H O Cr O SO 14H 2 4 2 2 7 Ag 2 2 2 8 3 + + + + + - - - + + (Cr O /Cr ) 1.33V 2 3 2 7 = - + Acidic condition: E (CrO /Cr(OH) ) 0.12V - 4 2 4 = - - Basic condition: E Cr2+(aq) Cr3+(aq)