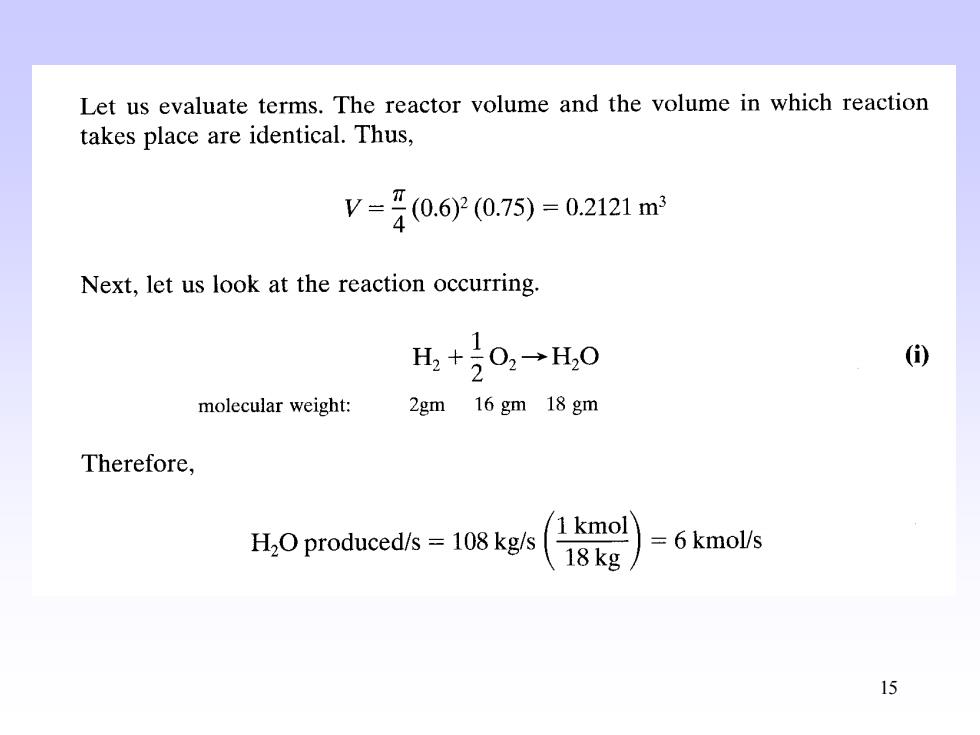
Let us evaluate terms.The reactor volume and the volume in which reaction takes place are identical.Thus, V=40.60.7)=02121m Next,let us look at the reaction occurring. 1 H,+20,→H,0 () molecular weight: 2gm 16 gm 18 gm Therefore, H2O produced/s =108 kg/s 1 kmol 18kg 6kmol/s 15
15

So from Eq.(i) H,used 6 kmol/s O2 used 3 kmol/s and the rate of reaction is 1 0.2121m3 6kmol=2.829×10 mol used (m3 of rocket)·s -r02= 0221m3kmo=1,415x10-mol 1 m3.s Note:Compare these rates with the values given in Figure 1.3. 16
16

EXAMPLE 1.2 THE LIVING PERSON A human being (75 kg)consumes about 6000 kJ of food per day.Assume that the food is all glucose and that the overall reaction is C6H1206+602→6C02+6H20,-4H,=2816kJ from air breathed out Find man's metabolic rate (the rate of living,loving,and laughing)in terms of moles of oxygen used per m3 of person per second. SOLUTION We want to find 1 dNo: mol O2 used () (m3 of person)s Let us evaluate the two terms in this equation.First of all,from our life experience we estimate the density of man to be P=1000kg n 17
17

Therefore,for the person in question 75kg Vem-1000kgm=0.075m Next,noting that each mole of glucose consumed uses 6 moles of oxygen and releases 2816 kJ of energy,we see that we need dNoz 6000 kJ/day 6 mol O2 =-12.8 mol O2 dt 2816 kJ/mol glucose 1 mol glucose day Inserting into Eq.(i) molO2used -r62= 1 12.8 mol O2 used 1day=0.002 0.075m3 day 24×3600s m3.s Note:Compare this value with those listed in Figure 1.3. 18
18

Product Reactor Regenerator Reducing Oxidizing atmosphere atmosphere Air Steam Air 19 Figure P1.3 The Exxon Model IV FCC unit
19