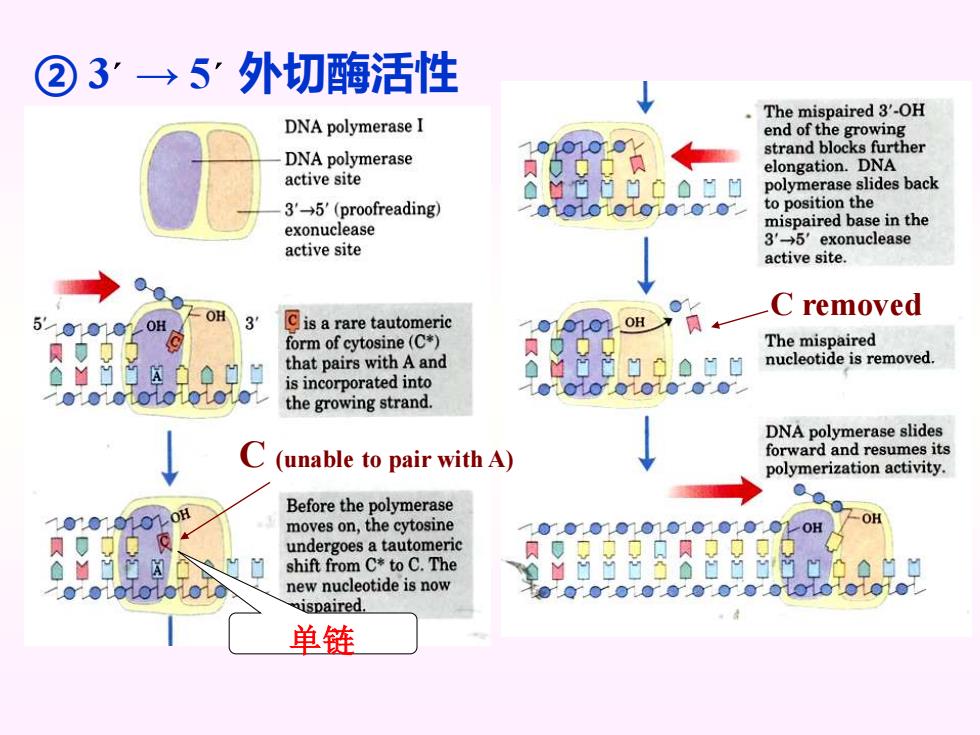
②3”→5外切酶活性 The mispaired 3'-OH DNA polymerase I end of the growing DNA polymerase strand blocks further elongation.DNA active site polymerase slides back 3'→5'(proofreading to position the exonuclease mispaired base in the 3'→5'exonuclease active site active site. C removed 5'000 OH OH 3 Cis a rare tautomeric OH form of cytosine(C*) The mispaired that pairs with A and 日0a日日 nucleotide is removed is incorporated into the growing strand DNA polymerase slides C (unable to pair with A) forward and resumes its polymerization activity. Before the polymerase 0 moves on,the cytosine OH undergoes a tautomeric shift from C*to C.The new nucleotide is now 00Y000000001001010 ispaired. 单链
C (unable to pair with A) C removed ② 3´ → 5´ 外切酶活性 单链
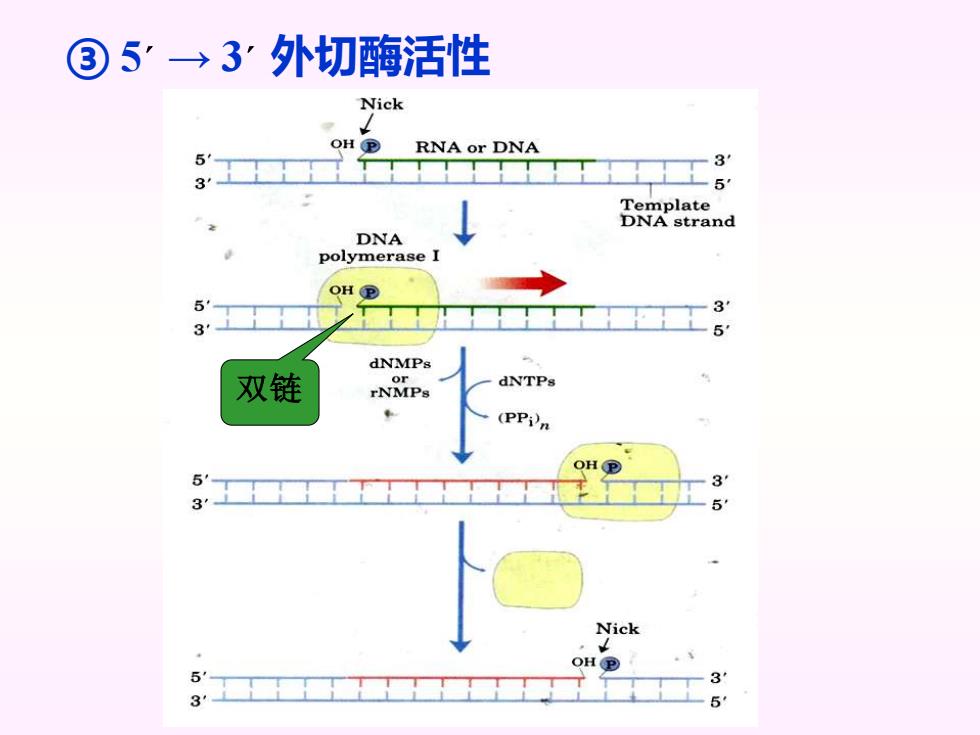
③5'→3:外切酶活性 Nick RNA or DNA 3 5 Template DNA strand DNA polymerase I OH P 3 5 dNMPs 双链 or dNTPs rNMPs (PPin 31 51 Nick OHP 31 3
③ 5´ → 3´ 外切酶活性 双链

Arthur Kornberg won the 1959 Nobel Prize in Medicine for his discovery of the mechanism in the biological synthesis of deoxyribonucleic acid (before Watson and Crick won theirs!)
Arthur Kornberg won the 1959 Nobel Prize in Medicine for his discovery of the mechanism in the biological synthesis of deoxyribonucleic acid (before Watson and Crick won theirs!)
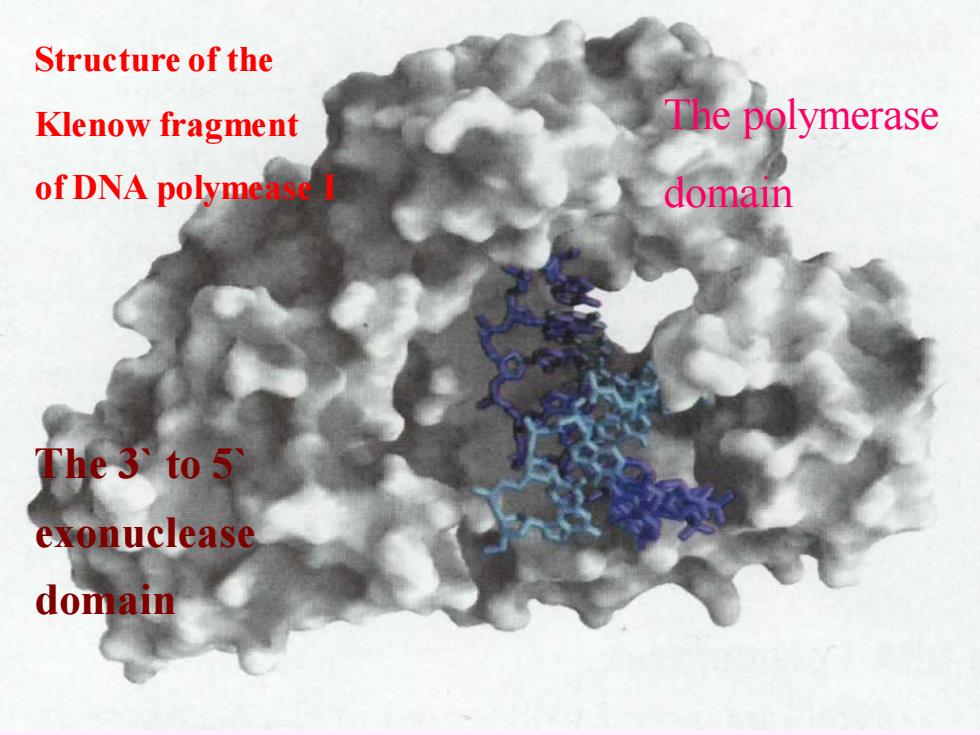
Structure of the Klenow fragment The polymerase of DNA polyme domain The 3 to 5 exonuclease domain
Structure of the Klenow fragment of DNA polymease I The polymerase domain The 3` to 5` exonuclease domain

(2)DNA聚合酶Ⅱ 单体酶,分子量120Kd。 令酶的催化活性: g ①具有5'→3聚合酶活性(活性很低) 。②具有3'→5外切酶活性 矿③无5'→3·外切酶活性
⑵ DNA聚合酶Ⅱ 单体酶,分子量120Kd。 酶的催化活性: ①具有 5´ → 3´聚合酶活性(活性很低) ②具有 3´ → 5´外切酶活性 ③无 5´ → 3´外切酶活性