
3.Coordination bond Formation condition:one atom has lone electron pair, while the other one has an empty orbit E.g NH BE] H F CHA ↓ π F C π H F 2s22p22s22p N:2S22P3 I▣(O)2p1 H':1S (C)2p H:1S1
3.Coordination bond Formation condition: one atom has lone electron pair, while the other one has an empty orbit + NH 4 [ ] − BF4 CO 2 2 2 s 2 p 2 4 2 s 2 p H − N − H H H F − B − F F F E.g: π C O π N: 2S 22P 3 H +: 1S 0 H: 1S 1 2 (C ) 2 p 4 ( O ) 2 p CH4 ?

9.1.3 Hybrid Orbits The principles of the hybrid orbit theory: .Hybridization is the mixing of nonequivalent atomic orbits with matching energies in an atom to generate a set of hybrid orbits. .The number of hybrid orbits generated is equal to the number of pure atomic orbits that participate in the hybridization process. .Hybrid orbits and pure atomic orbits have very different shapes
The principles of the hybrid orbit theory : •Hybridization is the mixing of nonequivalent atomic orbits with matching energies in an atom to generate a set of hybrid orbits. •The number of hybrid orbits generated is equal to the number of pure atomic orbits that participate in the hybridization process. •Hybrid orbits and pure atomic orbits have very different shapes. 9.1.3 Hybrid Orbits
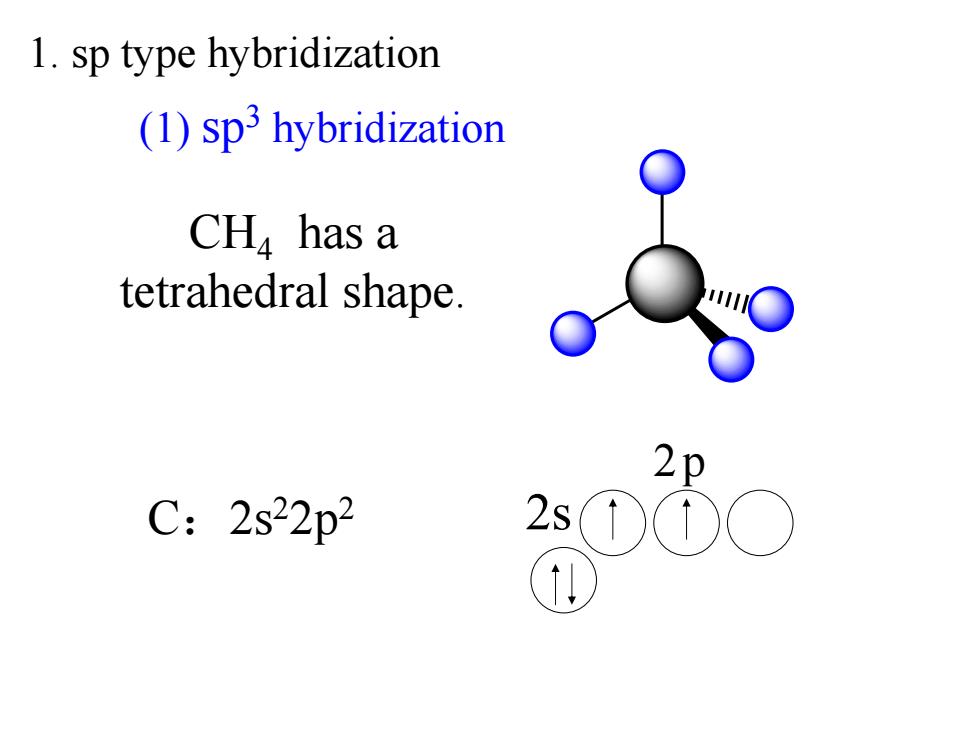
1.sp type hybridization (1)sp3 hybridization CH has a tetrahedral shape. 2p C:2s22p2 2s0
CH4 has a tetrahedral shape. 2p C:2s22p2 2s (1) sp3 hybridization 1. sp type hybridization
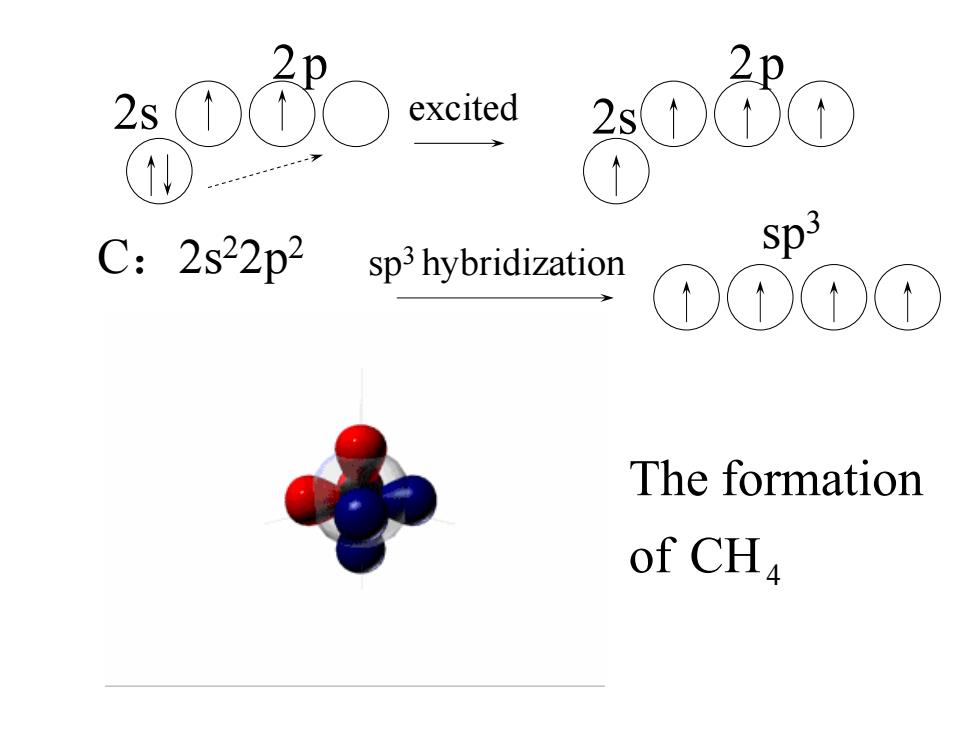
excited 2s1 C:2s22p2 sp3 hybridization Sp3 The formation of CHa
2s 2p CH4 of The formation 2p 2s sp3 C:2s22p2 excited sp3 hybridization

Four sp3 hybrid orbits:
Four sp3 hybrid orbits: