
(2)sp2 Hybridization F The molecular geometry of BFa is trigonal planar B F F B:2s22pl
2s 2p B: 2s22p1 (2) sp2 Hybridization B F F F The molecular geometry of BF3 is trigonal planar
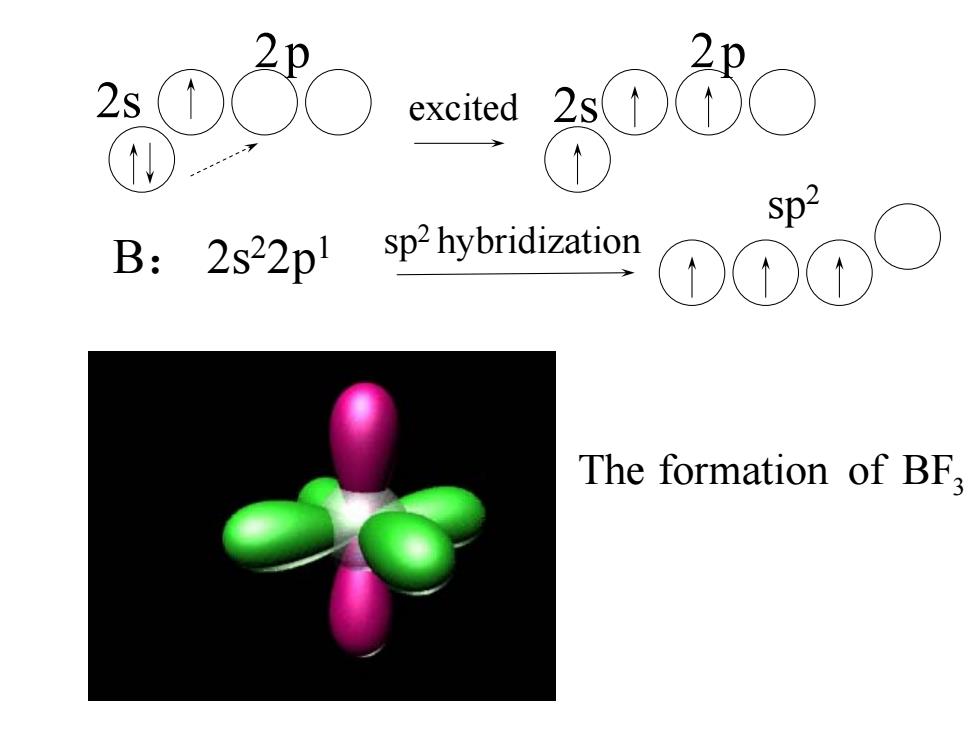
2p 2s sphyidio Sp2 B:2s22p 1 The formation of BF
of BF3 The formation 2s 2p 2p 2s sp2 sp2 hybridization B: 2s22p1 excited
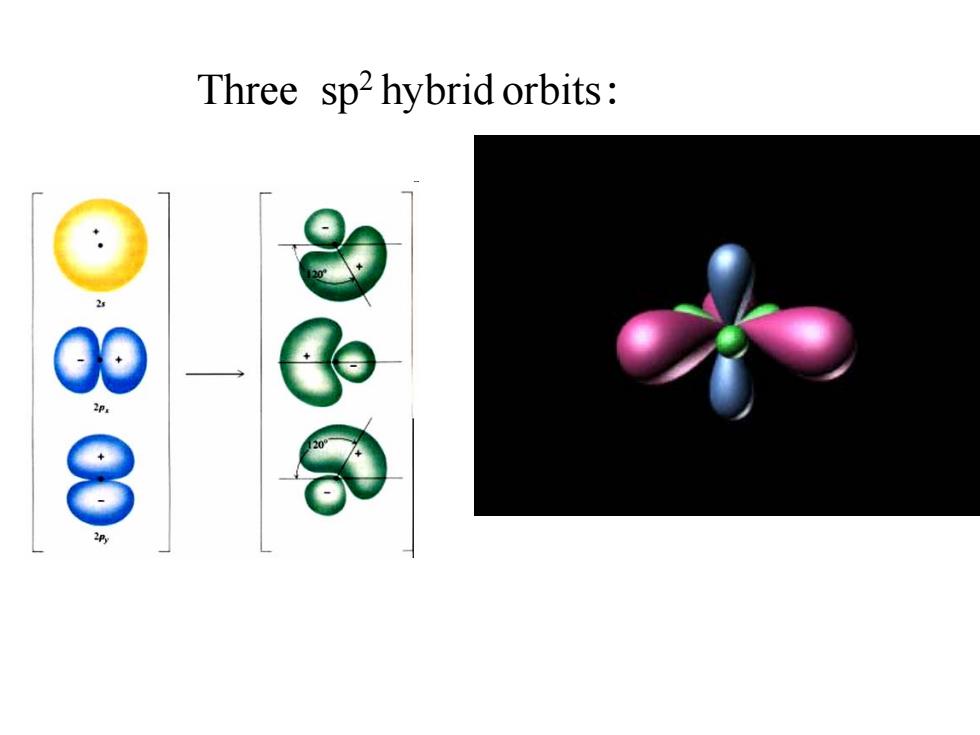
Three sp2 hybrid orbits: 2p 8
Three sp2 hybrid orbits:

(3)sp hybridization Be:2s2 The BeH,molecular is linear H-Be-H 220 excited 220 sp hybridization sp Be is sp-hybridized to form BeH,molecular
2s 2p Be:2s2 (3) sp hybridization The BeH2 molecular is linear H H Be 2s 2p 2p 2s sp sp hybridization Be is sp-hybridized to form BeH2 molecular excited
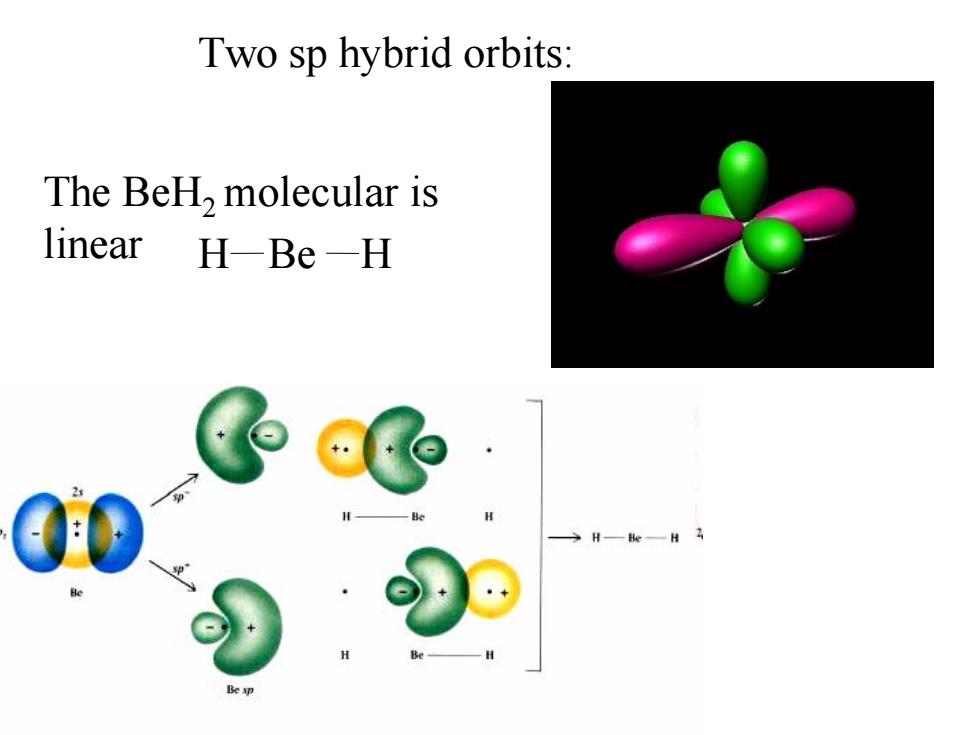
Two sp hybrid orbits: The BeH,molecular is linear H-Be-H H—eH
Two sp hybrid orbits: The BeH2 molecular is linear H H Be