
Chapter 7 Redox Reactions and Base of Electrochemistry X 7.1 The fundamental concepts of redox reactions x 7.2 Electrochemical cells x 7.3 Electrode potentials x 7.4 Application of electrode potentials
Chapter 7 Redox Reactions and Base of Electrochemistry §7.1 The fundamental concepts of redox reactions §7.2 Electrochemical cells §7.3 Electrode potentials §7.4 Application of electrode potentials

7.1 The fundamental concepts of redox reactions 7.1.1 Oxidation numbers -7.1.2 Balancing redox equations using ion-electron half-reaction method
§ 7.1 The fundamental concepts of redox reactions 7.1.1 Oxidation numbers 7.1.2 Balancing redox equations using ion-electron half-reaction method
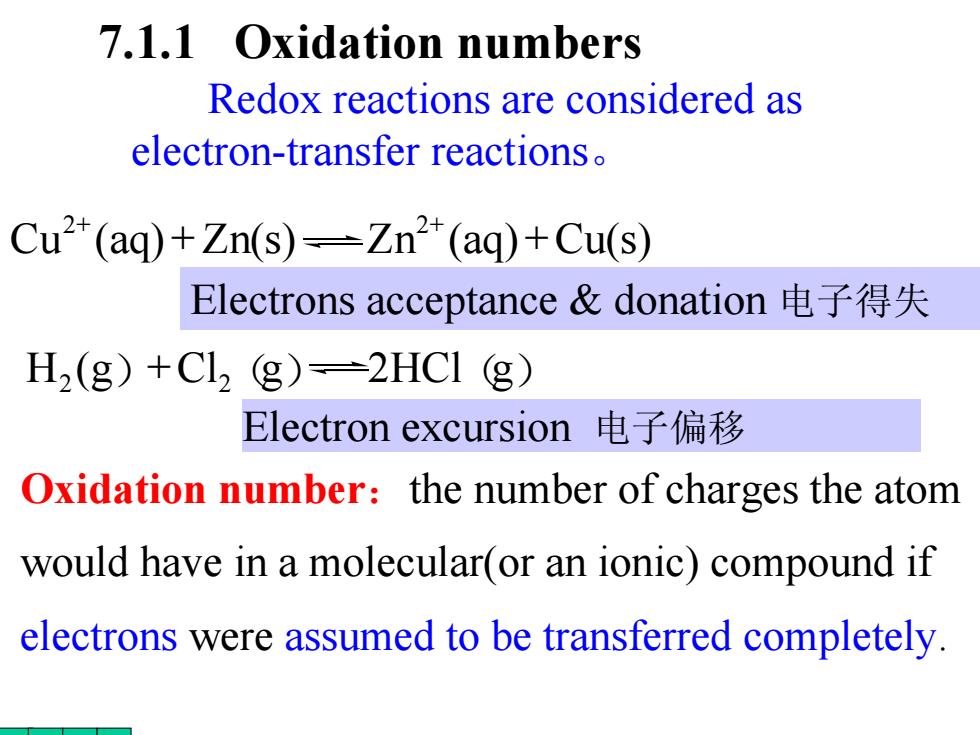
7.1.1 Oxidation numbers Redox reactions are considered as electron-transfer reactions. Cu2(aq)+Zn(s)-Zn(aq)+Cu(s) Electrons acceptance&donation电子得失 H2(g)+Cl2 g)=2HCI (g) Electron excursion电子偏移 Oxidation number:the number of charges the atom would have in a molecular(or an ionic)compound if electrons were assumed to be transferred completely
7.1.1 Oxidation numbers Oxidation number :the number of charges the atom would have in a molecular(or an ionic) compound if electrons were assumed to be transferred completely. Redox reactions are considered as electron-transfer reactions 。 Cu (aq ) Zn ( s ) Zn (aq ) Cu ( s ) 2 2 + + + + Electron excursion 电子偏移 H 2 ( g ) +Cl 2 (g ) 2HCl (g ) Electrons acceptance & donation 电子得失

The guideline for determining the oxidation number of an element in a substance In free elemental substances(单质),each atom has an oxidation number being zero. 2For ions consisting of a single atom,the oxidation number is equal to the charge on the ion 3The oxidation number of hydrogen is normally +1,except when it is bounded to metals in binary (二元)compound.In these cases,its oxidation number is -1
The guideline for determining the oxidation number of an element in a substance : ①In free elemental substances (单质 ), each atom has an oxidation number being zero. ②For ions consisting of a single atom, the oxidation number is equal to the charge on the ion. ③The oxidation number of hydrogen is normally +1, except when it is bounded to metals in binary (二元) compound. In these cases, its oxidation number is –1

4The oxidation number of oxygen in most compound is-2;but in peroxides(过氧化物) oxygen can have an oxidation number of-1. Oxygen can also have positive oxidation numbers when combined with fluorine.For example,the oxidation number of oxygen is +2 in OF2 and +1 in O2F2
④The oxidation number of oxygen in most compound is –2; but in peroxides(过氧化物) oxygen can have an oxidation number of –1. Oxygen can also have positive oxidation numbers when combined with fluorine. For example, the oxidation number of oxygen is +2 in OF2 and +1 in O2F2