
Chapter 12 The s-Block Elements 12.1 The general properties of the s-block elements X 12.2 The elementary substances קl2.3 The properties of the alkali and alkaline earth compounds X$12.4 The particularity of Li and Be The diagonal relationship
§12.1 The general properties of the s-block elements §12.4 The particularity of Li and Be The diagonal relationship §12.3 The properties of the alkali and alkaline earth compounds §12.2 The elementary substances Chapter 12 The s-Block Elements
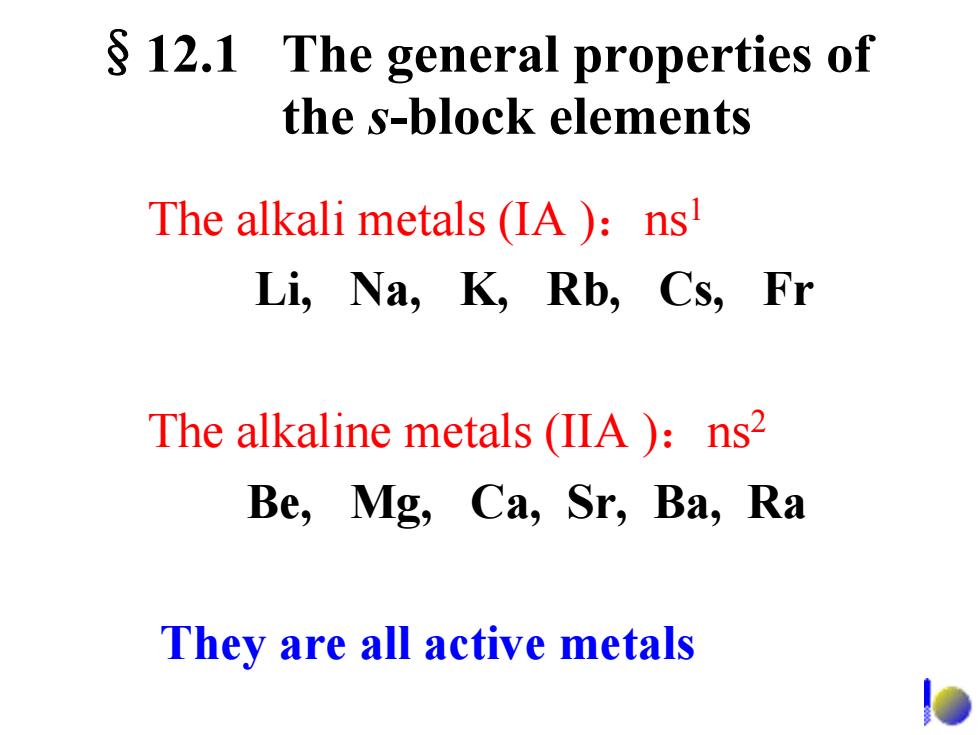
§12.1 The general properties of the s-block elements The alkali metals(IA )ns Li,Na,K,Rb,Cs,Fr The alkaline metals (IIA )ns2 Be,Mg,Ca,Sr,Ba,Ra They are all active metals e
§12.1 The general properties of the s-block elements The alkali metals (IA ):ns1 Li, Na, K, Rb, Cs, Fr The alkaline metals (IIA ):ns2 Be, Mg, Ca, Sr, Ba, Ra They are all active metals

IA IIA electronegativity Decreasing g ionization energy and Increasing metallicity Increasing atomic Li Be Na Mg K Ca Rb Sr radius Cs Ba reductibility Decreasing atomic radius Decreasing metallicity and reductibility Increasing ionization energy and electronegativity
electronegativity Decreasing ionization energy and Increasing metallicity and reductibility Increasing atomic radius Decreasing atomic radius Decreasing metallicity and reductibility Increasing ionization energy and electronegativity IA IIA Li Be Na Mg K Ca Rb Sr Cs Ba
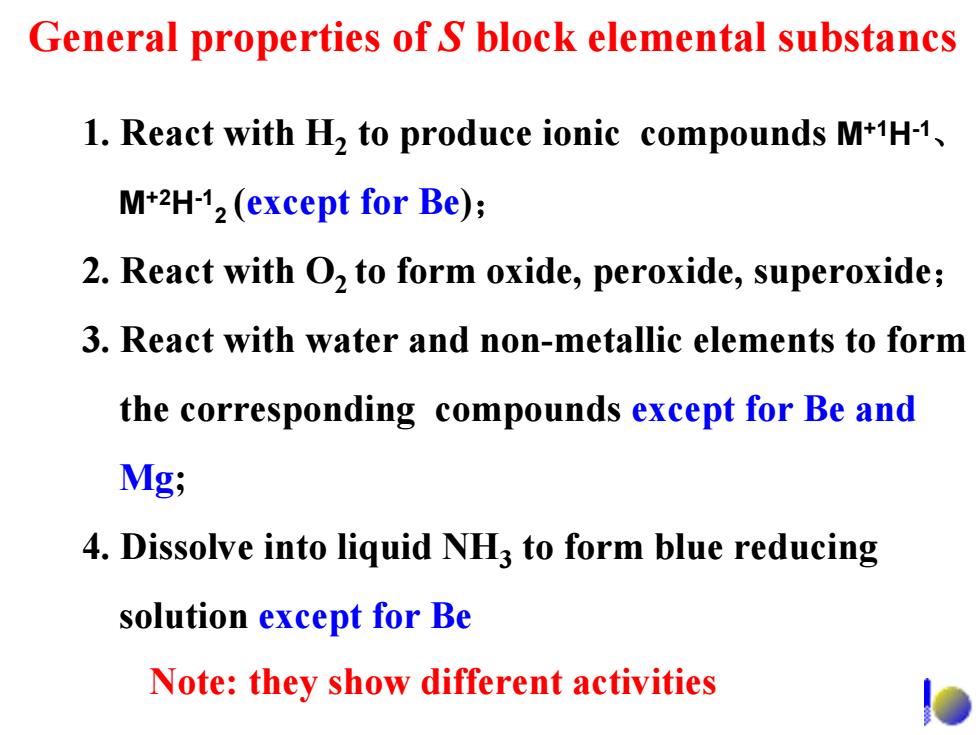
General properties of S block elemental substancs 1.React with H2 to produce ionic compounds M+1H-1, M+2H-12 (except for Be); 2.React with O,to form oxide,peroxide,superoxide; 3.React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4.Dissolve into liquid NH3 to form blue reducing solution except for Be Note:they show different activities
1. React with H2 to produce ionic compounds M+1H-1、 M+2H-12 (except for Be); 2. React with O2 to form oxide, peroxide, superoxide; 3. React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4. Dissolve into liquid NH3 to form blue reducing solution except for Be General properties of S block elemental substancs Note: they show different activities

S 12.2 The elementary substances 12.2.1 The properties of the elementary substances 1.Physical properties ☆Metallic luster; ☆Low densities; ☆Soft; ☆Low melting point; *Good conductor of heat and electricity Na K
§12.2.1 The properties of the elementary substances Na Li K 1.Physical properties §12.2 The elementary substances ☆Metallic luster; ☆Low densities; ☆Soft; ☆Low melting point; ☆Good conductor of heat and electricity