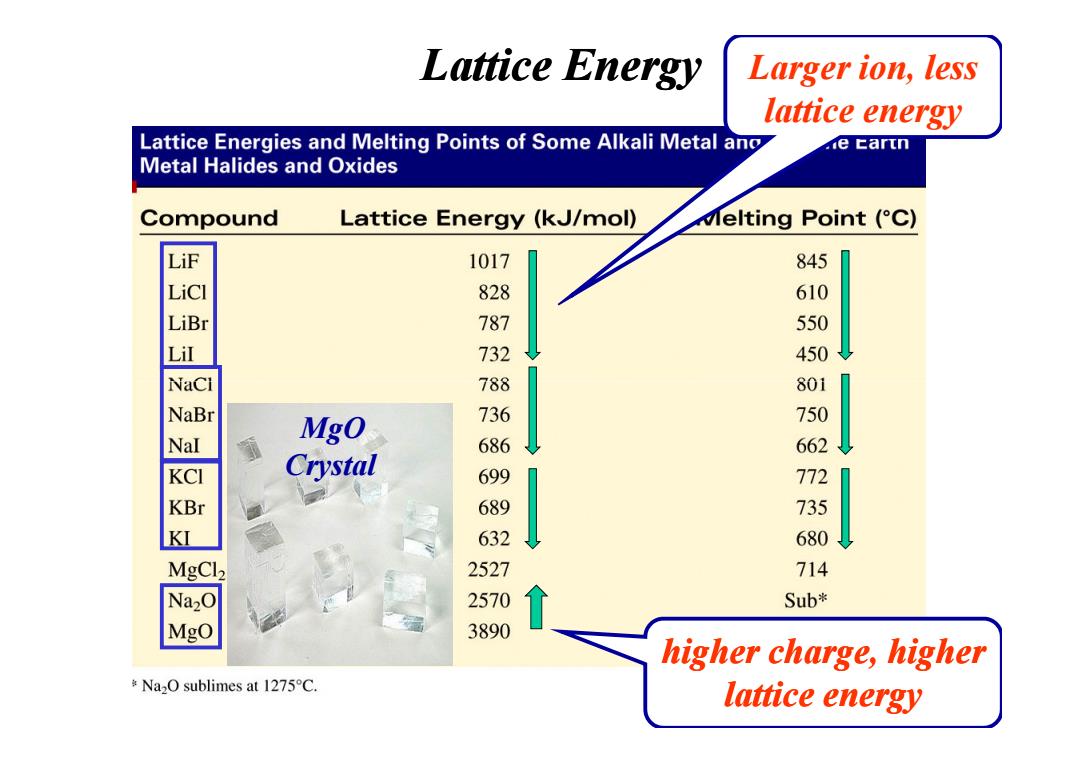
Lattice Energy Larger ion,less lattice energy Lattice Energies and Melting Points of Some Alkali Metal ano e tartn Metal Halides and Oxides Compound Lattice Energy(kJ/mol) Melting Point (C) LiF 1017 845 LiCI 828 610 LiBr 787 550 Lil 732 450 NaCi 788 801 NaBr 736 750 Nal Mgo 686 662 Crystal 699 772 689 735 KI 632 680 MgCl2 2527 714 Na2O 2570 Sub* MgO 3890 higher charge,higher Na2O sublimes at 1275C. lattice energy
Larger ion, less lattice energy Lattice Energy higher charge, higher lattice energy MgO Crystal
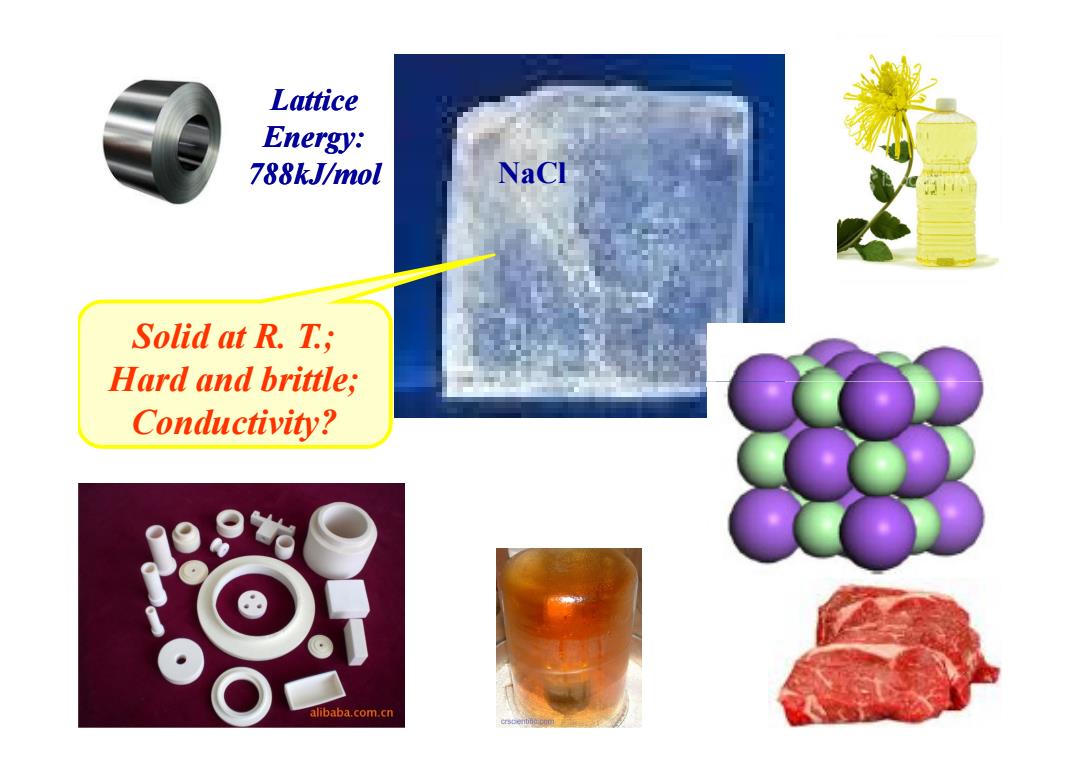
Lattice Energy: 788kJ/mol NaCl Solid at R.T.; Hard and brittle; Conductivity? alibaba.com.cn
Solid at R. T.; Hard and brittle; Lattice Energy: 788kJ/mol NaCl Hard and brittle; Conductivity?
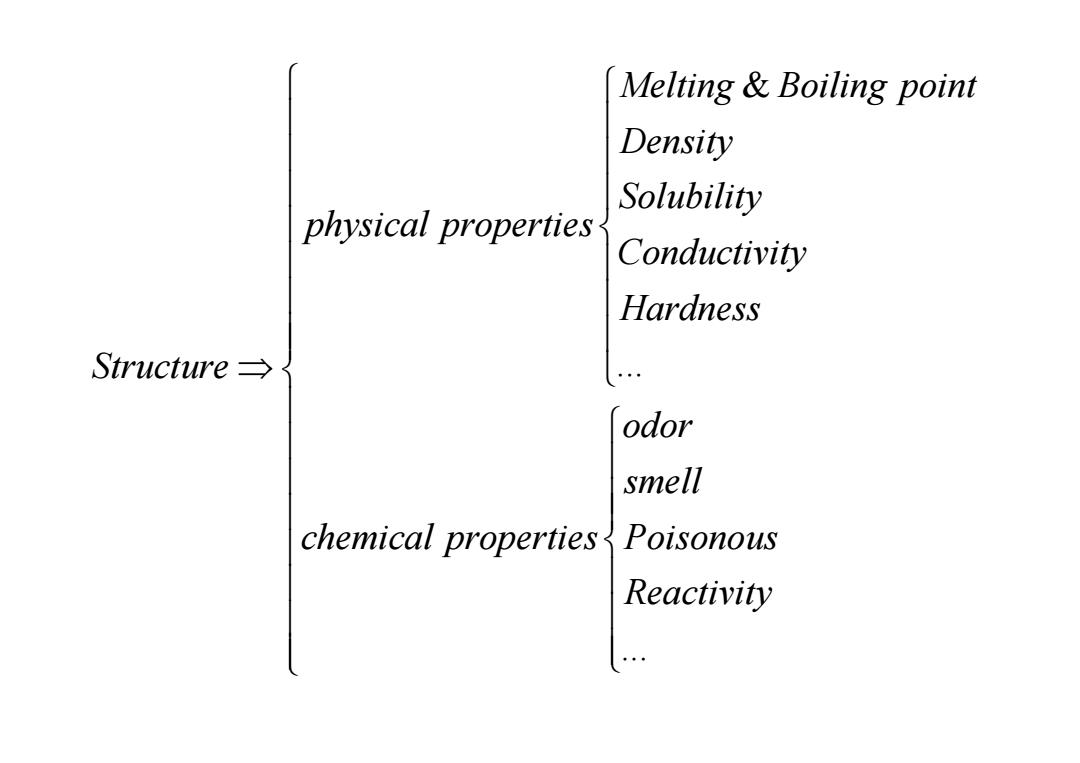
Melting Boiling point Density Solubility physical properties Conductivity Hardness Structure→ odor smell chemical properties Poisonous Reactivity
& ... Melting Boiling point Density Solubility physical properties Conductivity Hardness Structure ⇒ ... ... Structure odor smell chemical properties Poisonous Reactivity ⇒