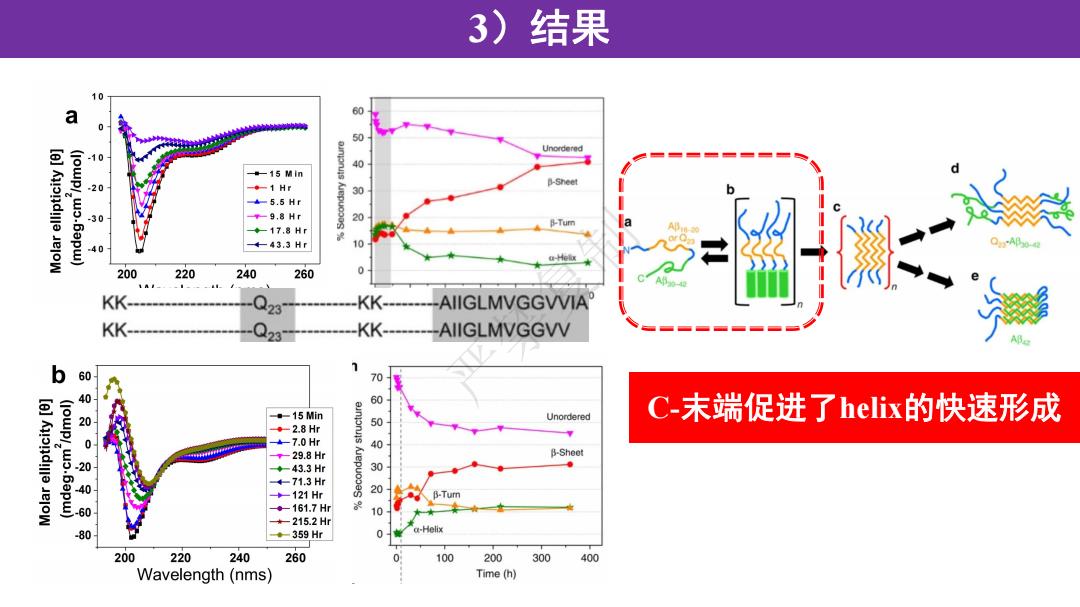
3)结果 a 60 50 10 -15 Min -◆-1Hr 500 B-Sheet +-5.5Hf -9.8 Hr 30 Tum ◆-17.8H 40 4-43.3Hr 10 (-H1e 200 220 240 260 0 KK- KK- AIIGLMVGGWVIA KK- KK- AIIGLMVGGWV b 7 60 70 40 15 Min 5 Unordered C-末端促进了helix的快速形成 ◆-2.8Hr 。 +-7.0Hr t-29.8Hr B-Sheet 43.3H 030 +-71.3H +-121Hr 0 60 。-161.7H 10 -215.2H -80 ◆-359Hr 0 a-Helix 200 220 240 260 0 100 200 300 400 Wavelength(nms) Time(h)
3)结果 严禁复制 C-末端促进了helix的快速形成
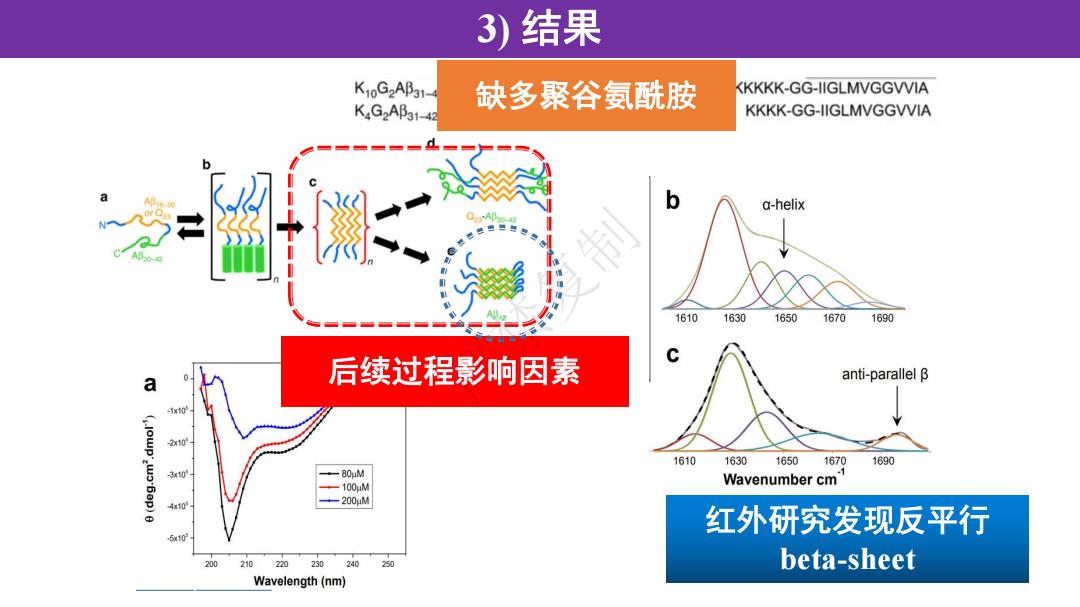
3)结果 K10G2AB31- KKKKK-GG-IIGLMVGGVVIA KG2Ap31-42 缺多聚谷氨酰胺 KKKK-GG-IIGLMVGGVVIA a-helix 16101630 1650 16701690 后续过程影响因素 anti-parallel B a 2x10 1610 1630 16501670 1690 310 +-80uM -100uM Wavenumber cm 4x10 -200uM 红外研究发现反平行 510 210220230240 20 beta-sheet Wavelength(nm)
3) 结果 缺多聚谷氨酰胺 后续过程影响因素 红外研究发现反平行 beta-sheet 严禁复制

蛋白质浴液检测红外- 最大的“敌人” 与安被打 水的红外光谱 0.8 0.6 如何解决? 0.4 0.2 FTIR spectroscopy Aggregates were collected at the end of aggregation reaction by centrifugation at 14,000 r.p.m.for 30 min.The pellet is washed once(PBS plus centrifugation),resuspended in~50ul of 1x PBS,and the suspension placed between two polished CaF2 windows using a BioCell module(BioTools,Inc.)on an ABB Prota-2x MB 3000 FTIR instrument.Spectra were collected at 4 cm-1 resolution (average of 400 scans)and corrected for buffer absorption until a flat baseline was obtained in the 1,700-1,800 cm region.Second derivative spectra for the amide-I region were calculated using PROTA software
蛋白质溶液检测红外 ------ 最大的“敌人” 如何解决? 严禁复制
课堂讨论 讨论1分离、纯化过程 讨论-2蛋白质一级结构测定 讨论3蛋白质二级结构检测 讨论4蛋白质三级结构检测 讨论5蛋白质高级结构解析简介
课堂讨论 讨论-1 分离、纯化过程 讨论-2 蛋白质一级结构测定 讨论-3 蛋白质二级结构检测 讨论-4 蛋白质三级结构检测 讨论-5 蛋白质高级结构解析简介 严禁复制