
热身活动:抽卡 1 2 4 5 6 7 8
1 2 3 4 热身活动:抽卡 5 6 7 8 严禁复制

热身活动:奔现的心情如何? (抽卡+弹幕分享) 1了土大文天关大 它反映出你来到线下 1 2 3 课堂的什么心情? 此刻你有什么想法? 5 6 7 8
1 2 3 4 热身活动:奔现的心情如何?(抽卡+弹幕分享) 它反映出你来到线下 课堂的什么心情? 此刻你有什么想法? 5 6 7 8 严禁复制
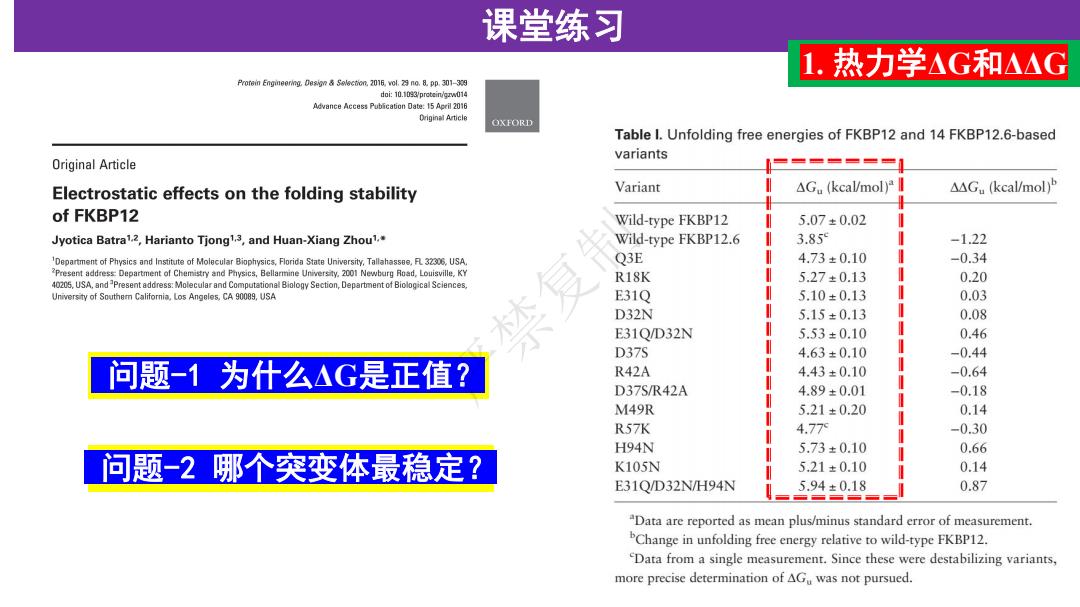
课堂练习 1.热力学△G和△△G Prorein Engineering.Solection.2016,vol.29 no.8.pp.301-339 doi:10.1093/protein/gzw014 Advance Access Pubfcation Date:15 April 2016 Driginal Article OXFORD Table I.Unfolding free energies of FKBP12 and 14 FKBP12.6-based variants Original Article Electrostatic effects on the folding stability Variant △G.(kcal/mol)a △△G.(kcal/mol)b of FKBP12 Wild-type FKBP12 5.07±0.02 Jyotica Batra1.2,Harianto Tjong1.3,and Huan-Xiang Zhou1. Wild-type FKBP12.6 3.85 -1.22 Department of Physics and Institute of Molecular Biophysics,Florida State University.Tallahassee.R32306,USA Q3E 4.73±0.10 -0.34 Present address Department of Chemistry and Physics,Bellarmine University.2001 Newburg Road,Louisville,KY R18K 5.27±0.13 0.20 40205,USA,and Present address:Molecular and Computational Biology Section,Departent of Biological Sciences, University of Southem California.Los Angeles,CA 90089,USA E31Q 5.10±0.13 0.03 D32N 5.15±0.13 0.08 E31Q/D32N 5.53±0.10 0.46 D37S 4.63±0.10 -0.44 问题-1为什么△G是正值? R42A 4.43±0.10 -0.64 D37S/R42A 4.89±0.01 -0.18 M49R 5.21±0.20 0.14 R57K 4.77 -0.30 H94N 5.73±0.10 0.66 问题-2哪个突变体最稳定? K105N 5.21±0.10 0.14 E31Q/D32N/H94N 5.94±0.18 0.87 Data are reported as mean plus/minus standard error of measurement. Change in unfolding free energy relative to wild-type FKBP12. Data from a single measurement.Since these were destabilizing variants, more precise determination of AGu was not pursued
问题-2 哪个突变体最稳定? 课堂练习 1. 热力学ΔG和ΔΔG 问题-1 为什么ΔG是正值? 严禁复制
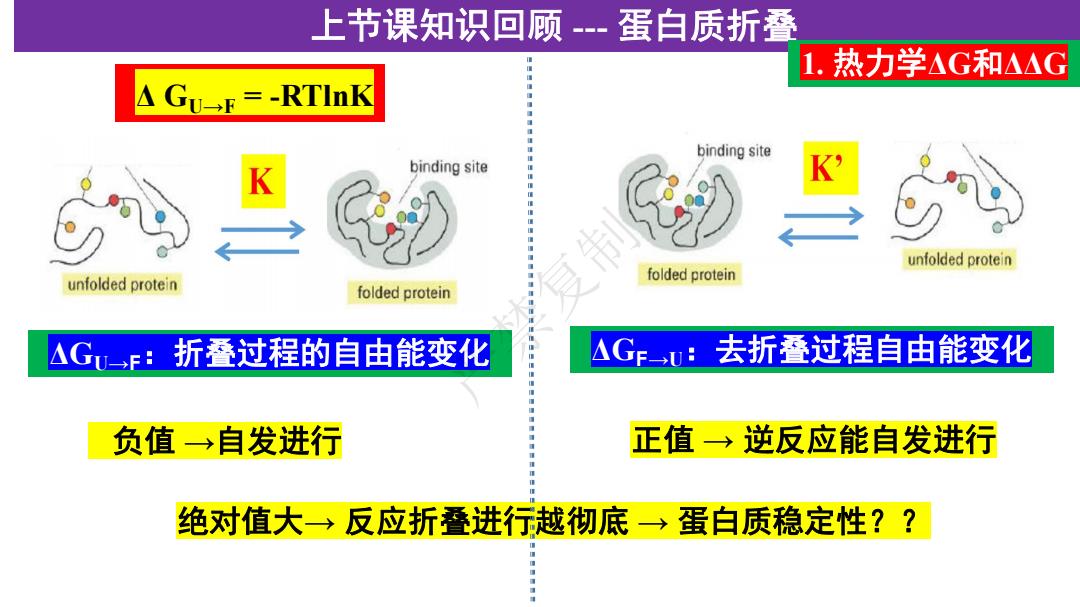
上节课知识回顾--蛋白质折叠 1.热力学AG和△△G △Gu→=-RTlnK binding site binding site unfolded protein unfolded protein folded protein folded protein △G→F:折叠过程的自由能变化 △GFu:去折叠过程自由能变化 负值→自发进行 正值→逆反应能自发进行 绝对值大→反应折叠进行越彻底→蛋白质稳定性??
上节课知识回顾 --- 蛋白质折叠 ΔGU→F:折叠过程的自由能变化 1. 热力学ΔG和ΔΔG 负值 →自发进行 绝对值大→ 反应折叠进行越彻底 → 蛋白质稳定性?? Δ GU→F = -RTlnK ΔGF→U:去折叠过程自由能变化 正值 → 逆反应能自发进行 严禁复制

课堂练习 1.热力学AG和△△G Prorein Engineering.Solection.2016,vol.29 no.8.pp.301-339 doi:10.1093/protein/gzw014 Advance Access Pubfcation Date:15 April 2016 Driginal Article OXFORD Table I.Unfolding free energies of FKBP12 and 14 FKBP12.6-based variants Original Article Electrostatic effects on the folding stability Variant △G.(kcal/mol)a △△G.(kcal/mol)P of FKBP12 Wild-type FKBP12 5.07±0.02 Jyotica Batra1.2,Harianto Tjong1.3,and Huan-Xiang Zhou1. Wild-type FKBP12.6 3.85 -1.22 Department of Physics and Institute of Molecular Biophysics,Florida State University.Tallahassee.R32306,USA Q3E 4.73±0.10 -0.34 Present address Department of Chemistry and Physics,Bellarmine University.2001 Newburg Road,Louisville,KY R18K 5.27±0.13 0.20 40205,USA,and Present address:Molecular and Computational Biology Section,Departent of Biological Sciences, University of Southem California.Los Angeles,CA 90089,USA E31Q 5.10±0.13 0.03 D32N 5.15±0.13 0.08 E31Q/D32N 5.53±0.10 0.46 D37S 4.63±0.10 -0.44 回答-1去折叠过程弘G正值 R42A 4.43±0.10 -0.64 D37S/R42A 4.89±0.01 -0.18 M49R 5.21±0.20 0.14 R57K 4.77 -0.30 H94N 5.73±0.10 0.66 ▣答-2E31Q/D32N/H94W K105N 5.21±0.10 0.14 E31Q/D32N/H94N 5.94±0.18 0.87 Data are reported as mean plus/minus standard error of measurement. Change in unfolding free energy relative to wild-type FKBP12. Data from a single measurement.Since these were destabilizing variants, more precise determination of AGu was not pursued
回答-2 E31Q/D32N/H94N 课堂练习 1. 热力学ΔG和ΔΔG 回答-1 去折叠过程ΔG正值 严禁复制