
§16.3 Cr Mo W 16.3.1 The elemental substances of Cr,Mo and W 16.3.2 The compounds of chromium
16.3.2 The compounds of chromium 16.3.1 The elemental substances of Cr, Mo and W §16.3 Cr Mo W

16.3.1 The elemental substances of Cr, Mo and W Group VIB elements:Cr,Mo,W Valence electron configuration: Cr,Mo:(n-1)d5ns1,W:5d46s2 1.The preparation of elemental Cr(the hardest metal It exists in the form of chromite Fe(CrO2)2 (铬铁矿)
Group VIB elements:Cr, Mo, W It exists in the form of chromite Fe(CrO2 )2 (铬铁矿) 1. The preparation of elemental Cr (the hardest metal ) 16.3.1 The elemental substances of Cr, Mo and W Valence electron configuration: Cr, Mo: (n-1)d5ns1 , W: 5d46s2
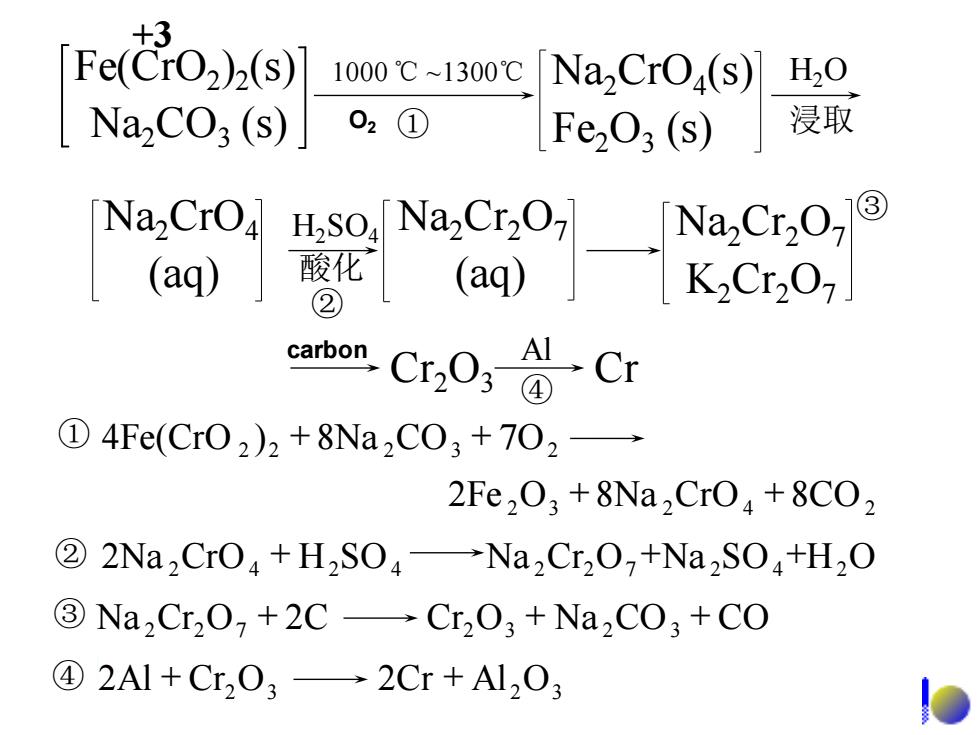
+3 Fe(CrO2)2(s) 1000℃~1300℃ NaCrO(s) H20 Na2CO3(s) 02① 1Fe03(s) 浸取 Na,CrO4 Na2Cr207 (aq) 酸化 ② carbon, Cr.O;Cr ①4Fe(Cr02)2+8Na2C03+702→ 2Fe,03+8Na2Cr04+8C02 ②2Na2Cr04+H2S04→Na2Cr20,+Na2S04+H20 3Na,Cr2O+2C-Cr2O3+Na2CO3+CO ④2Al+Cr03→2Cr+Al203
1000 ℃ ~1300℃ ① H2O 浸取 H2SO4 酸化 ② Cr2O3 Cr Al ④ Fe(CrO2 )2 (s) Na2CO3 (s) Na2CrO4 (s) Fe2O3 (s) Na2CrO4 (aq) Na2Cr2O7 (aq) Na2Cr2O7 K2Cr2O7 ③ 2Fe 2O3 8Na 2CrO4 8CO2 + + 2 2 8Na 2CO3 7O2 ① 4Fe(CrO ) + + ② 2Na 2CrO4 + H2SO4 Na 2Cr2O7 +Na 2SO4 +H2O 2Al Cr2O3 2Cr Al 2O3 ④ + + ③ Na 2Cr2O7 + 2C Cr2O3 + Na 2CO3 + CO +3 O2 carbon
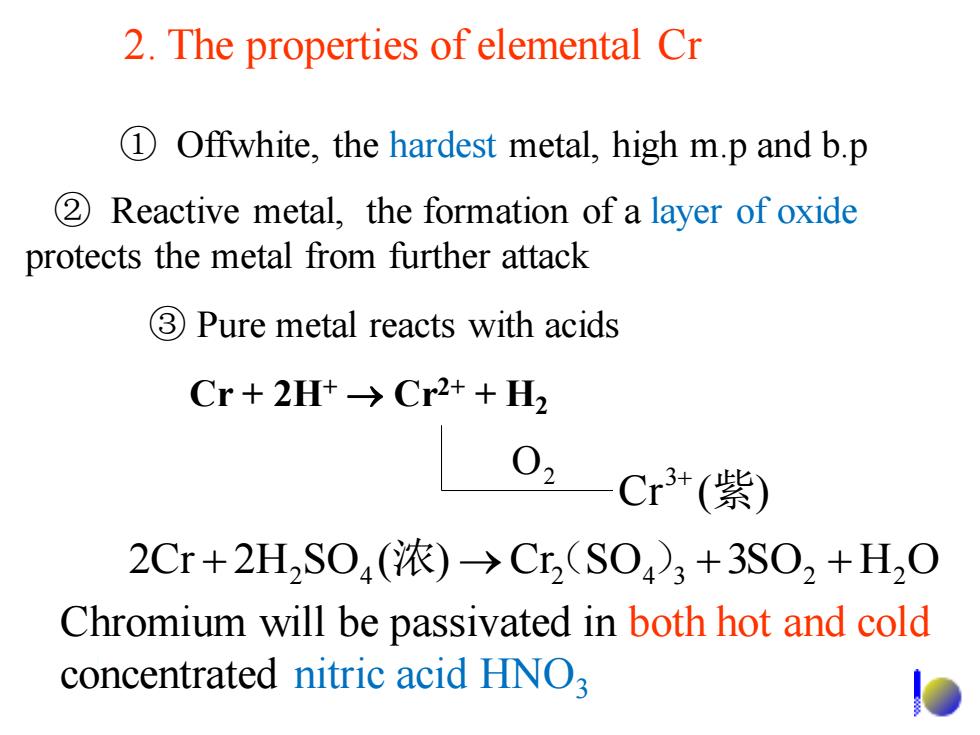
2.The properties of elemental Cr 1 Offwhite,the hardest metal,high m.p and b.p 2 Reactive metal,the formation of a layer of oxide protects the metal from further attack 3 Pure metal reacts with acids Cr+2Ht→Cr2++H2 02 Cr3+(紫) 2Cr+2H2SO4(浓)→Cr(S04)3+3SO2+H,O Chromium will be passivated in both hot and cold concentrated nitric acid HNO3
Chromium will be passivated in both hot and cold concentrated nitric acid HNO3 ③ Pure metal reacts with acids ② Reactive metal, the formation of a layer of oxide protects the metal from further attack ① Offwhite, the hardest metal, high m.p and b.p Cr ( ) 2 3+ 紫 O 2Cr + 2H2 SO4 (浓) →Cr(2 SO4 )3 +3SO2 + H2 O Cr + 2H+ → Cr2+ + H2 2. The properties of elemental Cr
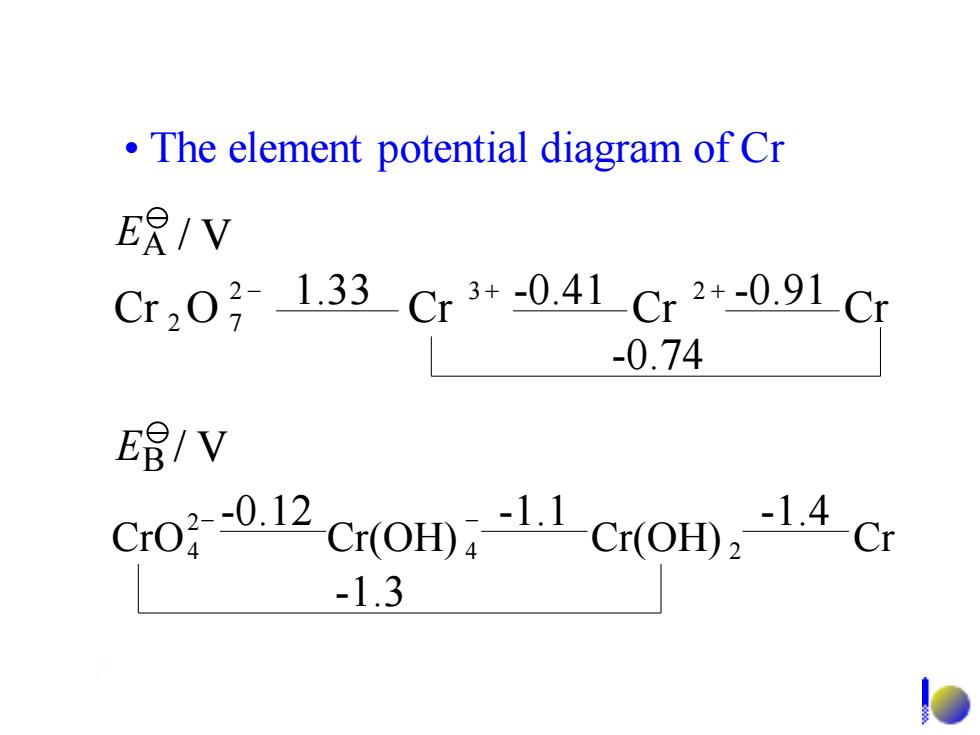
The element potential diagram of Cr ER/V Cr2031.33Cr3+-0.41Cr2+-0.91C -0.74 E8/V Cro0.12 Cr(OHD11 Cr(O)14Cr -1.3
• The element potential diagram of Cr Cr O Cr Cr Cr 2 3 2 2 7 − + + -0.74 1.33 -0.41 -0.91 CrO Cr(OH) Cr(OH) Cr 4 2 2 4 − -0.12 − -1.1 -1.4 -1.3 EB / V EA / V