
16.1.2 General physical properties High m.p and b.p Tungsten (W)has the highest m.p among the elemental substances ·High hardness The hardest metal:Cr ·High densities The most dense metal:Os Good conductor of heat and electricity,and good ductibility(延展性)
16.1.2 General physical properties • High m.p and b.p Tungsten (W) has the highest m.p among the elemental substances • High hardness The hardest metal: Cr • High densities The most dense metal: Os • Good conductor of heat and electricity, and good ductibility(延展性)
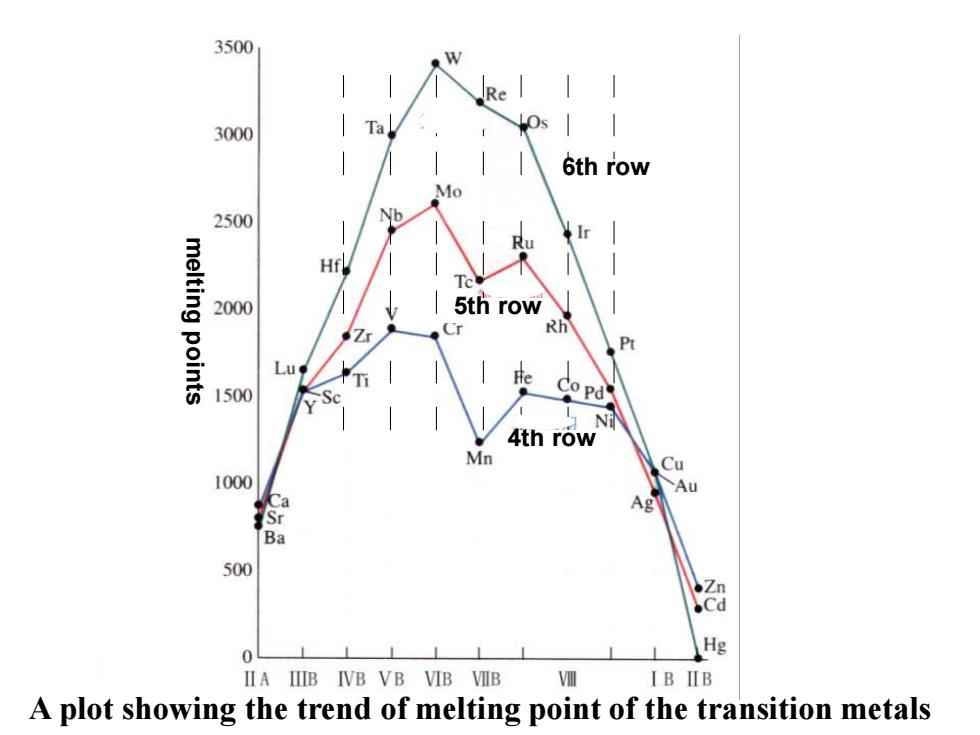
3500 Re 3000 Ta 6th row Mo 2500 Nb Ir melting points Hf Tc 2000 5th row Cr Rh 1500 Co Pd Ni 4th row Mn Cu 1000 Au Ag Ba 500 ●Zn .Cd 0 Hg IIA IIIB IVB VB VIB VIIB VI IB IIB A plot showing the trend of melting point of the transition metals
A plot showing the trend of melting point of the transition metals melting points 6th row 5th row 4th row
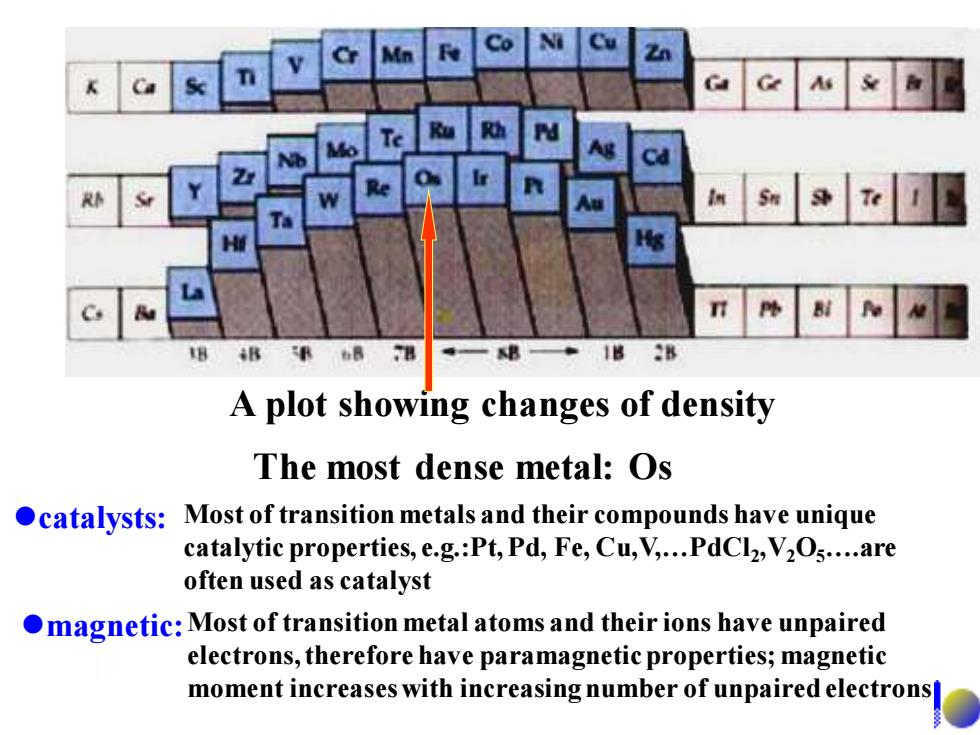
8 A plot showing changes of density The most dense metal:Os catalysts:Most of transition metals and their compounds have unique catalytic properties,e.g.:Pt,Pd,Fe,Cu,V,...PdCl2,V2Os....are often used as catalyst magnetic:Most of transition metal atoms and their ions have unpaired electrons,therefore have paramagnetic properties;magnetic moment increases with increasing number of unpaired electrons
A plot showing changes of density The most dense metal: Os ⚫catalysts: Most of transition metals and their compounds have unique catalytic properties, e.g.:Pt, Pd, Fe, Cu,V,…PdCl2 ,V2O5….are often used as catalyst ⚫magnetic:Most of transition metal atoms and their ions have unpaired electrons, therefore have paramagnetic properties; magnetic moment increases with increasing number of unpaired electrons
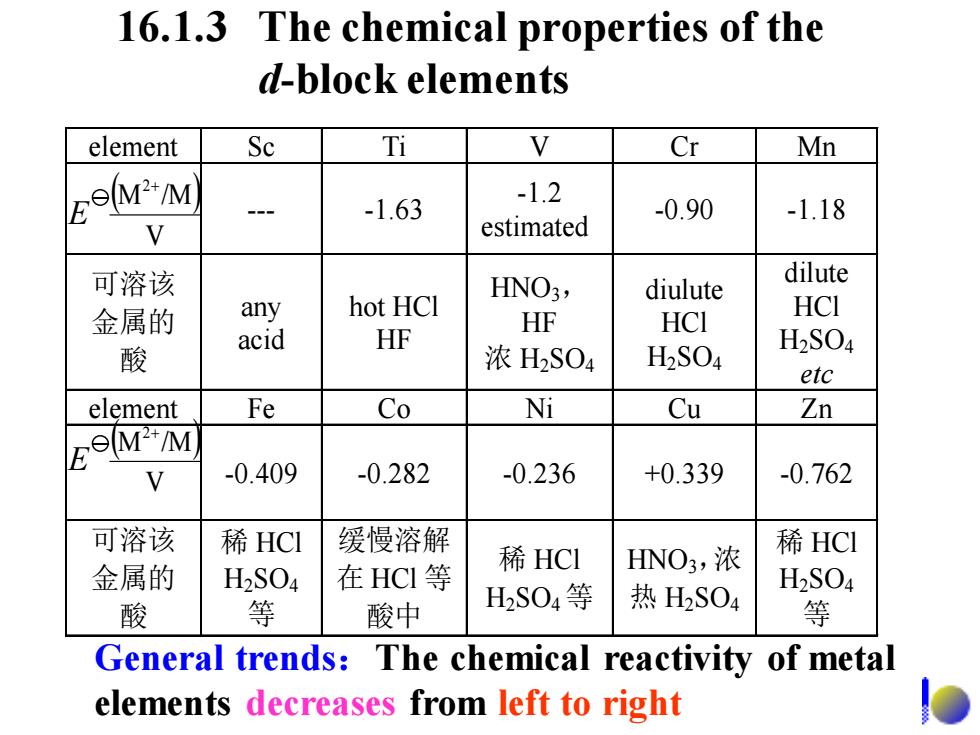
16.1.3 The chemical properties of the d-block elements element Sc Ti Cr Mn MM -1.63 -1.2 -0.90 -1.18 V estimated 可溶该 HNO3, diulute dilute 金属的 any hot HCI HF HCI acid HCI HF 酸 浓H2S04 H2S04 H2S04 etc element Fe Co Ni Cu Zn FoMM N -0.409 -0.282 -0.236 +0.339 -0.762 可溶该 稀HCI 缓慢溶解 稀HCl HNO3,浓 稀HCI 金属的 H2SO4 在HCI等 H2SO4 酸 等 酸中 H2S04等 热H2SO4 等 General trends:The chemical reactivity of metal elements decreases from left to right
16.1.3 The chemical properties of the d-block elements General trends:The chemical reactivity of metal elements decreases from left to right element Sc Ti V Cr Mn --- -1.63 -1.2 estimated -0.90 -1.18 可溶该 金属的 酸 any acid hot HCl HF HNO3, HF 浓 H2 SO4 diulute HCl H2 SO4 dilute HCl H2 SO4 etc element Fe Co Ni Cu Zn -0.409 -0.282 -0.236 +0.339 -0.762 可溶该 金属的 酸 稀 HCl H2 SO4 等 缓慢溶解 在 HCl 等 酸中 稀 HCl H2 SO4 等 HNO3,浓 热 H2 SO4 稀 HCl H2 SO4 等 ( ) V M /M2+ E ( ) V M /M2+ E
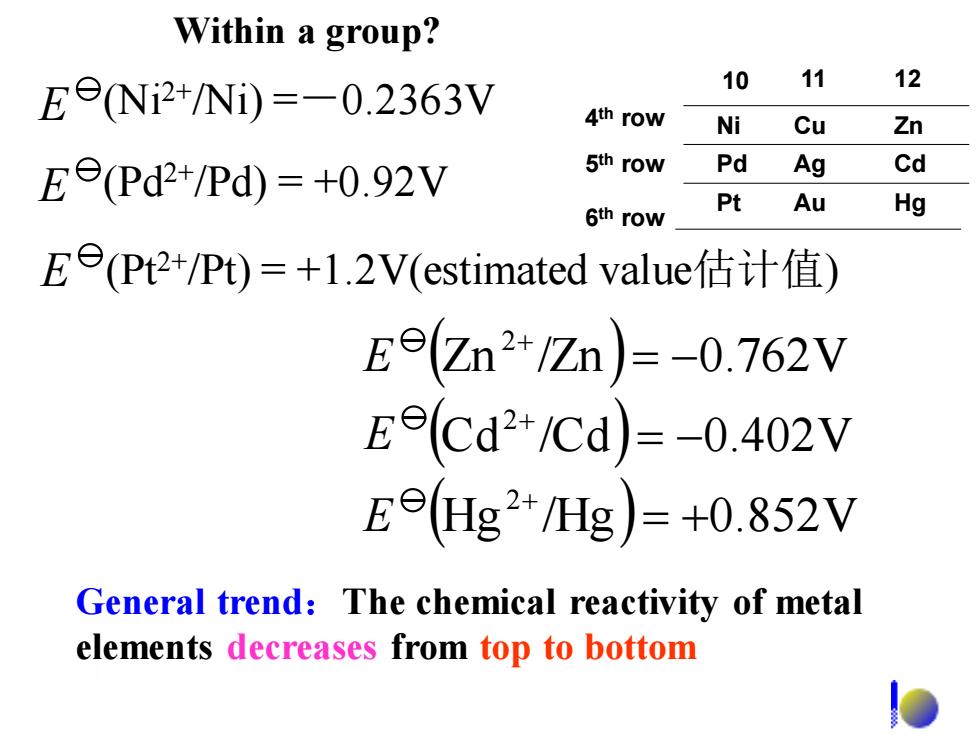
Within a group? E9Ni2+/Ni)=-0.2363V 10 11 12 4th row Ni Cu Zn Ee(Pd2+/Pd)=+0.92V 5th row Pd Ag Cd Pt 6th row Au Hg Ee(Pt2+/Pt)=+1.2V(estimated value估计值) Ee(Zn2+/Zn)=-0.762V Eecd2+/Cd)=-0.402V Ee(Hg2+/Hg)=+0.852V General trend:The chemical reactivity of metal elements decreases from top to bottom
General trend:The chemical reactivity of metal elements decreases from top to bottom ( ) ( ) (Hg /Hg ) 0.852V Cd /Cd 0.402V Zn /Zn 0.762V 2 2 2 = + = − = − + + + E E E E (Ni2+/Ni) =-0.2363V E (Pd2+/Pd) = +0.92V E (Pt2+/Pt) = +1.2V(estimated value估计值) Within a group? 4 th row 5 th row 6 th row Ni Pd Pt Cu Ag Au Zn Cd Hg 10 11 12