
6.2 Forming and dissolving of precipitates 6.2.1 The solubility product rule -6.2.2 The common ion effect and salt effect -6.2.3 The effect of pH on solubility -6.2.4 The effect of complex ions formation on solubility
§ 6.2 Forming and dissolving of precipitates 6.2.4 The effect of complex ions formation on solubility 6.2.3 The effect of pH on solubility 6.2.2 The common ion effect and salt effect 6.2.1 The solubility product rule
6.2.1 The solubility product rule A B (s)-nAm"(aq)+mB"-(aq) J={c(Am+)}"{c(B"-)}"m The solubility product rule:The reaction quotient criterion for precipitationsolubility equilibria equilibrium proceeds in reverse direction p,equilibrium state,saturated solution <Kp,equilibrium proceeds in forward direction
6.2.1 The solubility product rule m n n m { (A )} { (B )} + - J = c c A B (s) nA (aq) mB (aq) m n n m + - + The solubility product rule: The reaction quotient criterion for precipitation—solubility equilibria ☆ J >Ksp , equilibrium proceeds in reverse direction ☆ J = Ksp , equilibrium state, saturated solution ☆ J < Ksp , equilibrium proceeds in forward direction
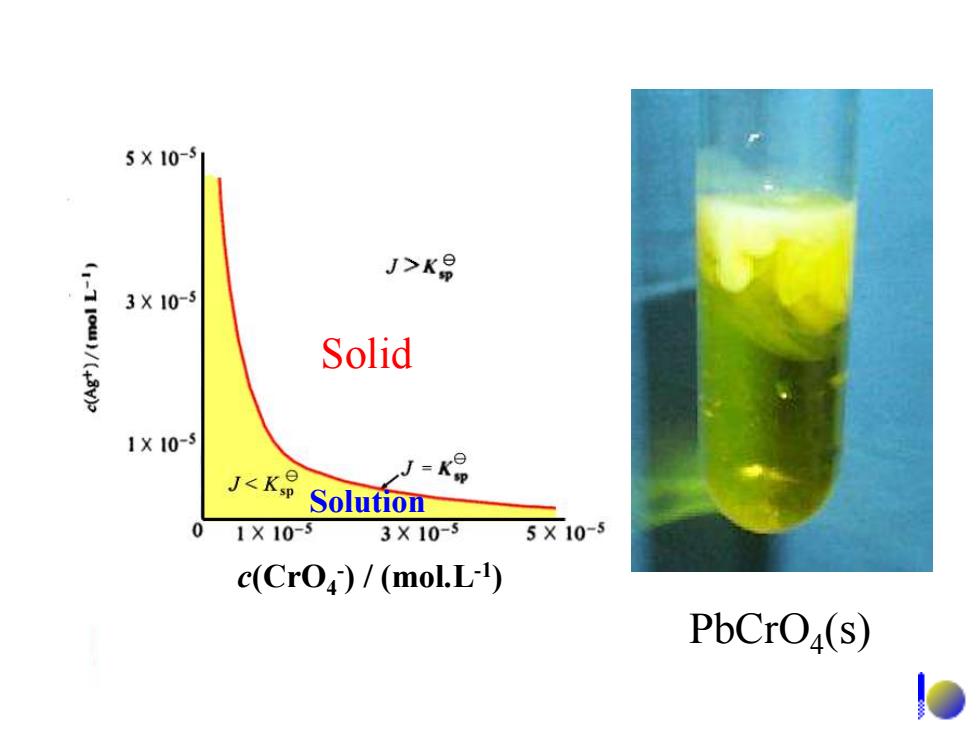
5X10-5 J>K号 3X10-5 Solid 1X10-5 J<K J=k Solution 01×10-3 3X10-3 5X10-5 c(Cro)/(molL-1) PbCrO(s)
PbCrO4 (s) Solid Solution c(CrO4 - ) / (mol.L-1 )
Application of the solubility product rule Example:BaCO(s)-Ba2(aq)+CO (aq) ①Adding acids:2Ht+CO}→H,O+CO, c(CO3)↓J↓J<K Adding acids is beneficial to dissolving BaCO 2Adding BaCl2 or Na2CO3 c(Ba2+)个orc(C0})个J个J>K BaCO,will precipitate out
Example: 2 2 2 2H +CO3 H O+CO + - BaCO3 will precipitate out. ② Adding BaCl2 or Na2CO3 ① Adding acids: BaCO (s) Ba (aq) CO (aq) 2 3 2 3 + - + Adding acids is beneficial to dissolving BaCO3 2 3 c(CO ) J J < - Ksp or 2 3 c(CO ) J J > - + (Ba ) 2 c Ksp Application of the solubility product rule

Example:Supposing 10.0L of 0.010mol-L-BaCl2 is added to 40.0L of a solution that contains SO2-ion with a concentration of 6.0X 10-4 mol-L-at 25C. Does BaSO precipitate?If so,how many grams of BaSO,are formed?What is the concentration of SO2- in the final solution? K9=1.1x100 Answer: c(S0)= 6.0×104×40.0 =4.8×104molL 50.0 c(Ba2+)= 0.010×10. 2=2.0x103molL 50.0
Example:Supposing 10.0L of 0.010mol·L-1 BaCl2 is added to 40.0L of a solution that contains SO4 2- ion with a concentration of 6. 0×10-4 mol·L-1 at 25℃. Does BaSO4 precipitate? If so, how many grams of BaSO4 are formed? What is the concentration of SO4 2- in the final solution? Answer: 4 1 4 2 o 4 4.8 1 0 mol L 5 0.0 6.0 1 0 4 0.0 (SO ) - - - - = c = 2 3 1 o 2.0 10 mol L 50.0 0.010 10.0 (Ba ) + - - = c = 10 sp 1.1 10- K =