
上游克通大粤 SHANGHAI JIAO TONG UNIVERSITY Chapter 8.Phase Rule p/kPa B 水 dp △H dTeg Te9△V (液相) 22090 dp △fsHm Tm①-ms】 △H,a吧 冰 dp▣ dTeg (固相) 蒸发线 1ap0 101.35 汽 0.611 (气相 升华线 0 273.16373.15 647.29 T/K
Chapter 8. Phase Rule
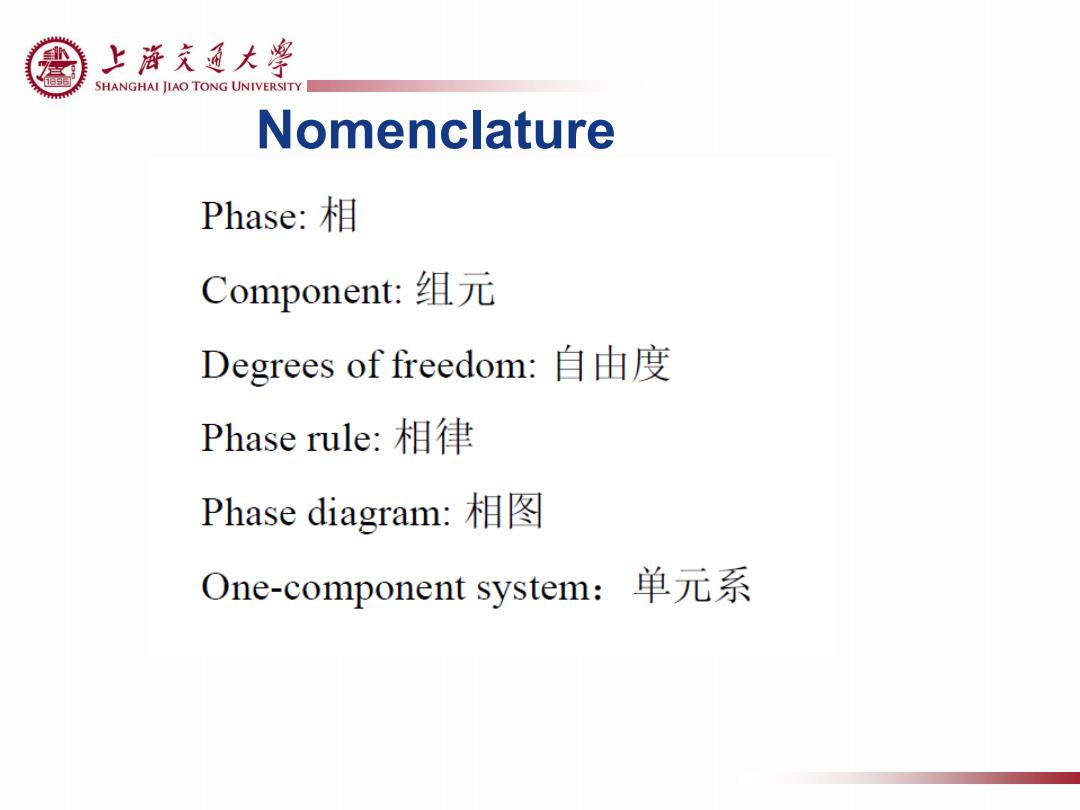
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Nomenclature Phase:相 Component:组元 Degrees of freedom:自由度 Phase rule:相律 Phase diagram:相图 One-component system:单元系
Nomenclature

上浒充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phases Phase:a homogeneous,physically distinet,and mechanically separable portion of matter present in a nonhomogeneous physical chemical system. <Webster's Dictionary> GHAIJIAO TONG UNIVE nm
Phases

上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Phases(2) in thermodynamics,chemically and physically uniform or homogeneous quantity of matter that can be separated mechanically from a nonhomogeneous mixture and that may consist of a single substance or of a mixture of substances.The three fundamental phases of matter are solid,liquid,and gas (vapour),but others are considered to exist,including crystalline,glassy,amorphous,and plasma phases. Thermodynamics Kinetics
Phases (2) Thermodynamics Kinetics

上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phases(3) Matter is considered to form one homogeneous phase if its atomic or molecular dispersion is uniform;e.g.,a glass of water containing dissolved salt,sugar,and a dye constitutes only a single liquid phase.If hundreds of grains of sand were added,all the grains together would constitute only a single additional (solid)phase. The different phases of a pure substance bear a fixed relationship to one another in terms of temperature and pressure.Thus,if the pressure on some liquids is raised,they will freeze at a higher temperature.This relationship is extremely important in industrial as well as scientific work. Salt water; Pure water with two pieces of ice on the surface; Gold sands and sands; alloys
Phases (3) Salt water; Pure water with two pieces of ice on the surface; Gold sands and sands; alloys