11.I Gas,Liquid and Solid on the Molecular Level Cool or compress Cool Heat or Heat reduce pressure Gas Liquid Crystalline solid Total disorder Disorder Ordered arrangement much empty space particles or clusters: particles in fixed position particles:free to move are free to move particles:close together particles:far apart particles close together
11.1 Gas, Liquid and Solid on the Molecular Level Total disorder much empty space particles: free to move particles: far apart particles : close together are free to move particles or clusters : Disorder particles : close together particles : in fixed position Ordered arrangemen t

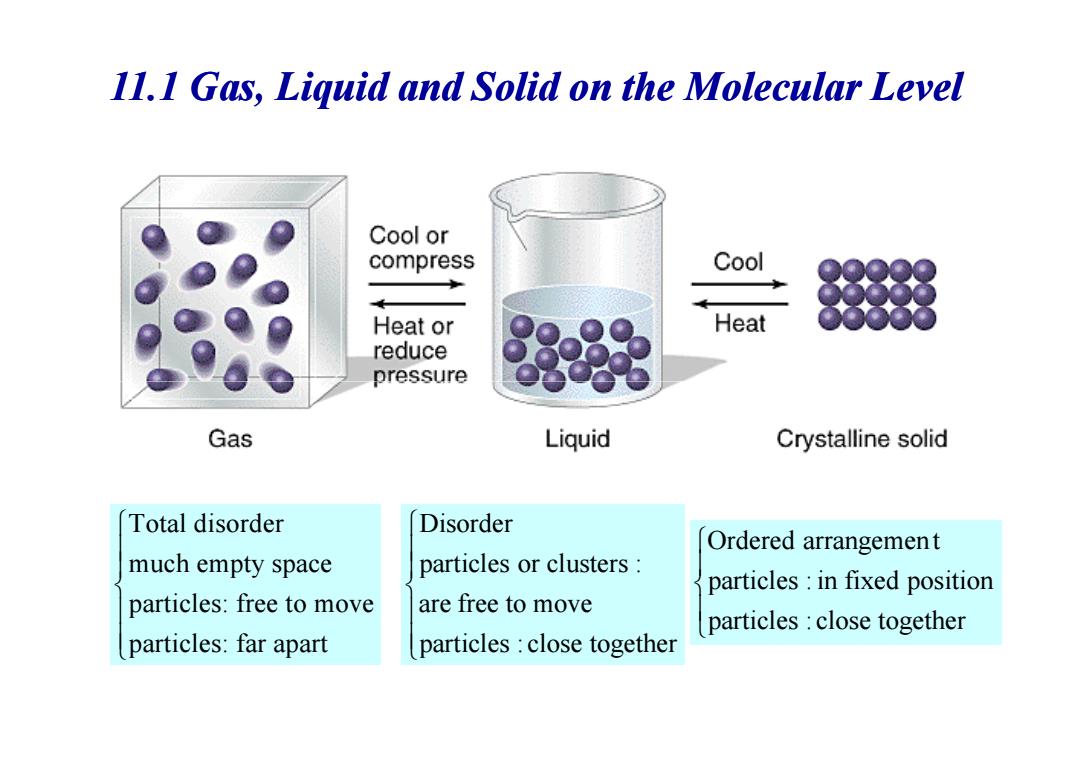
Gas,Liquid and Solid on the Molecular Level 3 states of matter are closely related to Intermolecular forces Gaseous state: Kinetic energy completely overcomes intermolecular forces. Particles move freely unrelated to each other. ·Liquid state:. Intermolecular forces hold particles close together. Kinetic energy keeps them moving around each other. ●Solid state:. Intermolecular forces hold particles in fixed positions -Kinetic energy keeps them vibrating in place
• Gaseous state: – Kinetic energy completely overcomes intermolecular forces. – Particles move freely & unrelated to each other. Gas, Liquid and Solid on the Molecular Level 3 states of matter are closely related to Intermolecular forces E >>E kinetic attraction • Liquid state: – Intermolecular forces hold particles close together. – Kinetic energy keeps them moving around each other. • Solid state: – Intermolecular forces hold particles in fixed positions. –Kinetic energy keeps them vibrating in place. E < E kinetic attraction

Intermolecular Forces I (van der Waals forces) The van der Waals force is the attractive or repulsive forces between molecules 偶极子 1.Ion-Dipole Attraction: Interaction between an ion and a dipole (a) (b) The Strongest in all intermolecular forces
1. Ion-Dipole Attraction: • Interaction between an ion and a dipole Intermolecular Forces I (van der Waals forces Waals forces) The van The van der Waals force Waals force is the attractive or repulsive forces between molecules 偶极子 • Interaction between an ion and a dipole The Strongest in all intermolecular forces σ - σ +

Intermolecular Forces I (van der Waals forces) 1.Ion-Dipole Attraction: Interaction between an ion and a dipole NaCl aqueous solution
1. Ion-Dipole Attraction: • Interaction between an ion and a dipole Intermolecular Forces I (van der Waals forces) NaCl aqueous solution

Intermolecular Forces i (van der Waals forces) Weaker than 偶极 2.Dipole-Dipole Attraction: ion-dipole forces Interaction between polar molecules Dipole-dipole forces among polar molecules cause them to have "preferred"orientations in the liquid or solid states
2. Dipole-Dipole Attraction Dipole Attraction: Interaction between polar molecules Intermolecular Forces I (van der Waals forces) Weaker than ion-dipole forces 偶极 Dipole-dipole forces among polar molecules cause them to have “preferred” orientations in the liquid or solid states