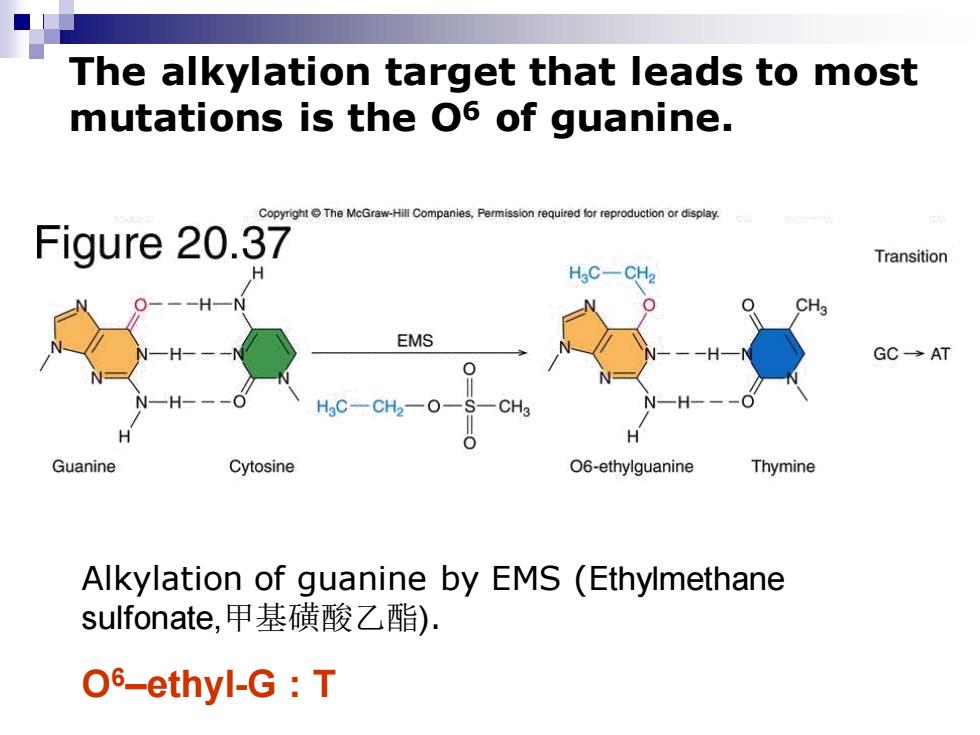
The alkylation target that leads to most mutations is the O6 of guanine. Copyright e The McGraw-Hill Compar ssion required fo reproduction or display. Figure 20.37 Transition H3C一CH EMS GC→AT -CH -0 CH Guanine Cytosine 06-ethylguanine Thymine Alkylation of guanine by EMS (Ethylmethane sulfonate,甲基磺酸乙酯). O6-ethyl-G T
Alkylation of guanine by EMS (Ethylmethane sulfonate,甲基磺酸乙酯). O6–ethyl-G : T The alkylation target that leads to most mutations is the O6 of guanine
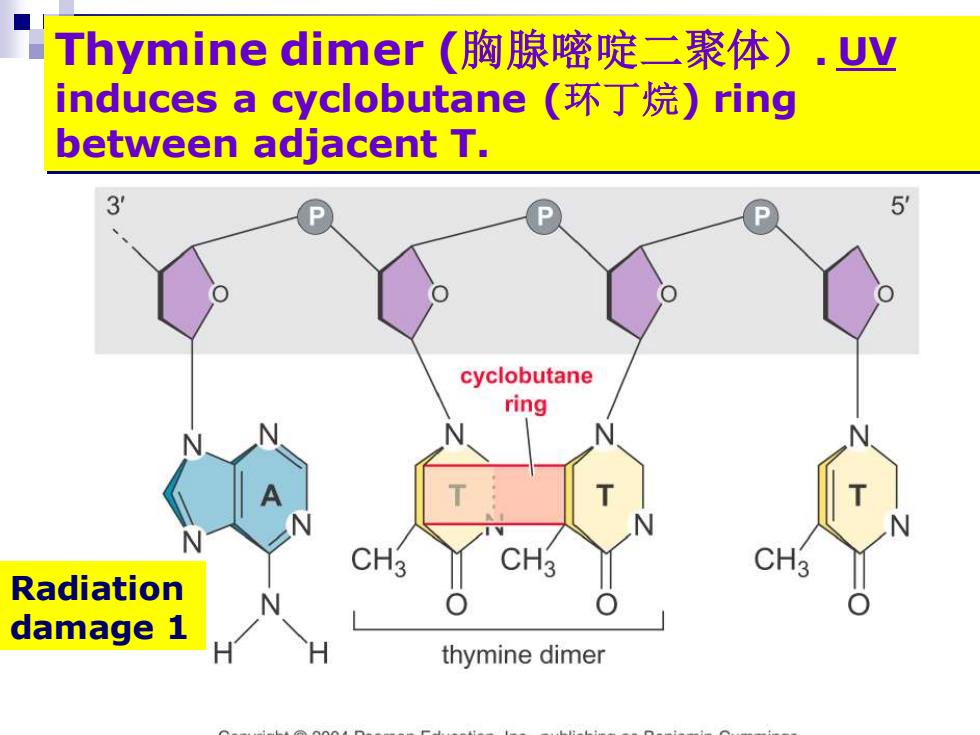
Thymine dimer(胸腺嘧啶二聚体).uy induces a cyclobutane(环丁烷)ring between adjacent T. 3 P 5 cyclobutane ring CH3 Radiation damage 1 thymine dimer
Thymine dimer (胸腺嘧啶二聚体). UV induces a cyclobutane (环丁烷) ring between adjacent T. Radiation damage 1

Gamma radiation and X-rays (ionizing radiation,电离辐射)cause double-strand breaks (DSB)and are particularly hazardous (hard to be repaired). Radiation damage 2
Gamma radiation and X-rays (ionizing radiation,电离辐射) cause double-strand breaks (DSB) and are particularly hazardous (hard to be repaired). Radiation damage 2
1.3 Mutations are also caused by base analogs(碱基类似物and intercalating agents(嵌入剂) >Base analogs:similar enough to the normal bases to be processed by cells and incorporated into DNA during replication. >But they form base pair differently, leading to mispairing during replication. >The most mutagenic base analog is 5-bromoUracil(5-BrU)(5-溴尿嘧啶), which is the analog of thymine
1.3 Mutations are also caused by base analogs (碱基类似物) and intercalating agents(嵌入剂) ➢Base analogs: similar enough to the normal bases to be processed by cells and incorporated into DNA during replication. ➢But they form base pair differently, leading to mispairing during replication. ➢The most mutagenic base analog is 5-bromoUracil (5-BrU) (5-溴尿嘧啶), which is the analog of thymine
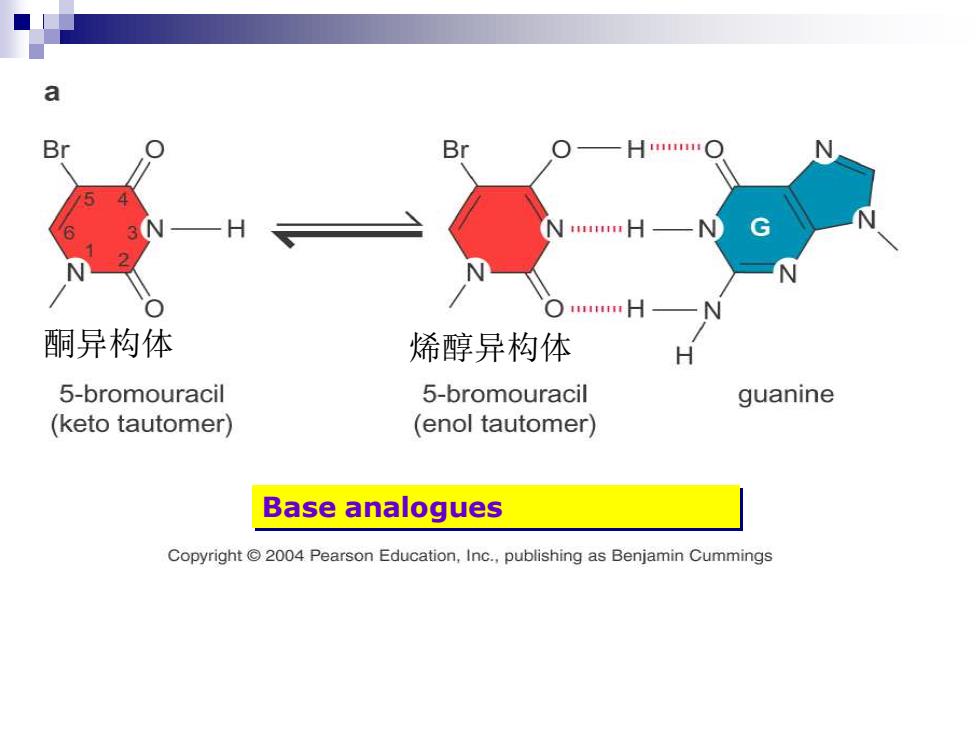
a Br Br —H○ ○#H 酮异构体 烯醇异构体 5-bromouracil 5-bromouracil guanine (keto tautomer) (enol tautomer) Base analogues Copyright @2004 Pearson Education,Inc.,publishing as Benjamin Cummings
Base analogues 酮异构体 烯醇异构体