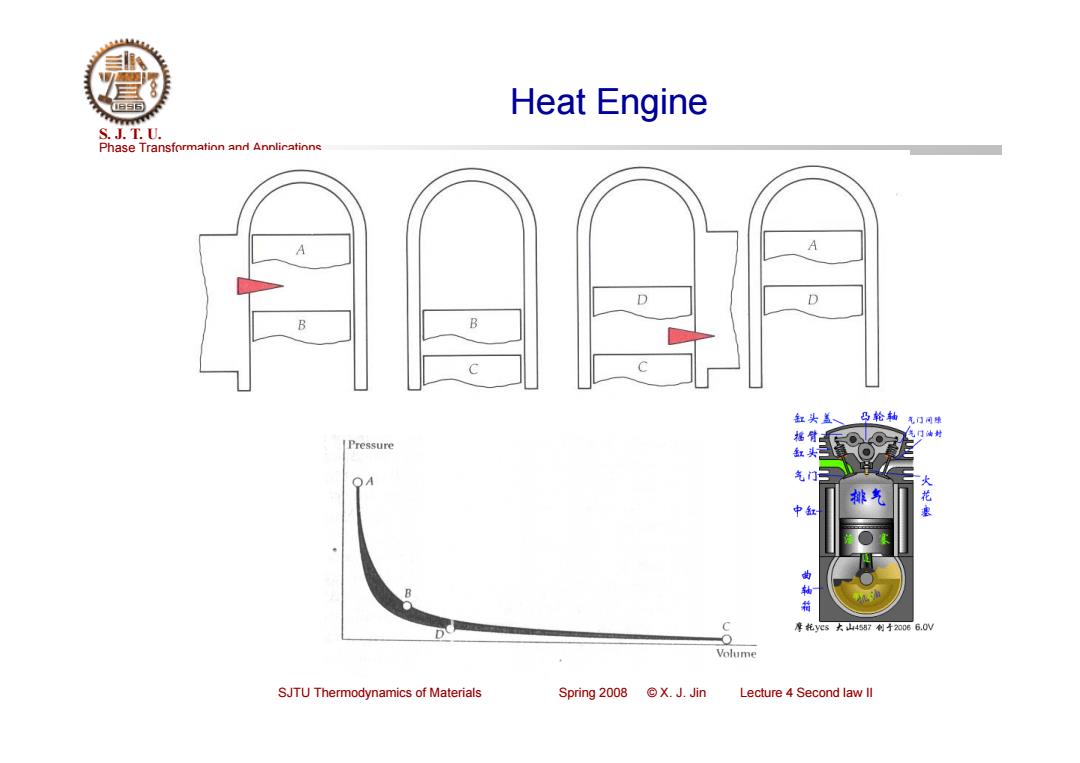
Heat Engine S.J.T.0. Phase Transformation and Annlicatinns A D D B 缸头盖、 凸轮轴气口 福臂 气门封 Pressure 缸头 气门 OA 中缸 曲 箱 C 摩花yes大山4557制寸20066.0W Volume SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Heat Engine
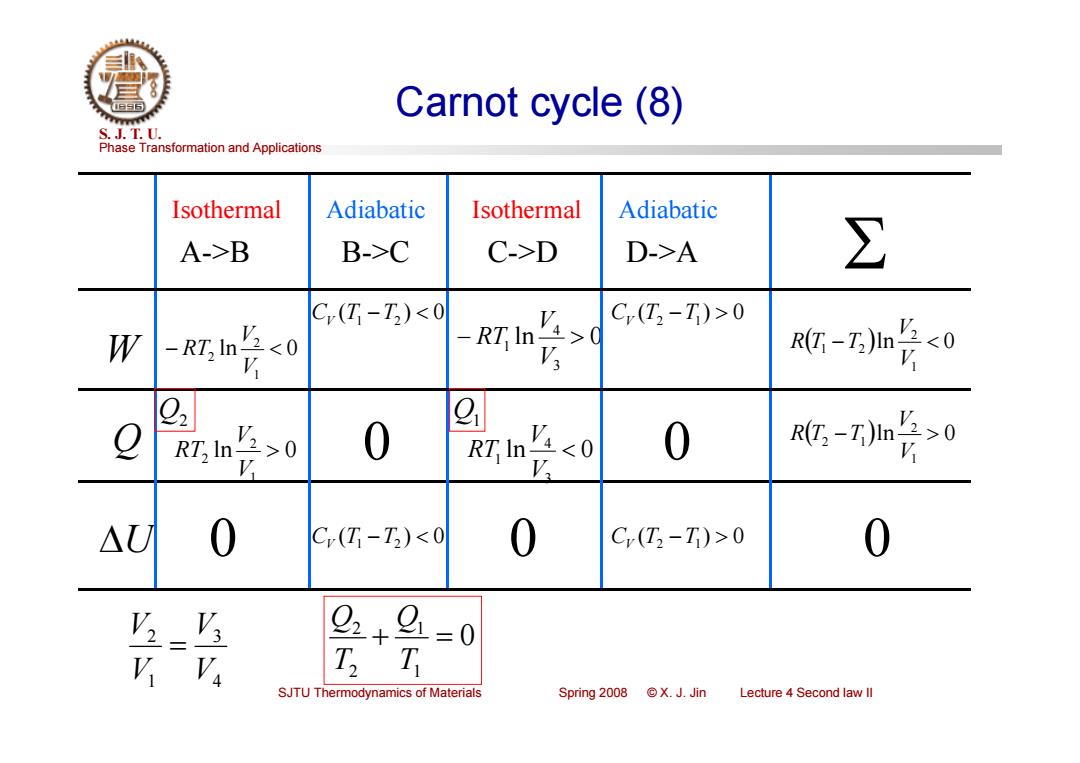
Carnot cycle (8) S.J.T.0. Phase Transformation and Applications Isothermal Adiabatic Isothermal Adiabatic A->B B->C C->D D->A ∑ C(T-T)<0 C(T2-T)>0 W -RT In V20 RT In V Rt-Z)n点<0 g RT In V2>0 0 RT In V,0 0 :-7m长0 △U 0 C(T-T)<0 0 C(T2-T)>0 0 2+9 =0 SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Carnot cycle (8) W ln 0 3 4 1 V V Q ln 0 RT 1 2 2 V V RT ln 0 1 2 2 V V RT U 0 A->B B->C C->D D->A 0 Isothermal Isothermal Adiabatic Adiabatic ln 0 3 4 1 V V RT 0 0 CV (T1 T2 ) 0 CV (T2 T1) 0 CV (T1 T2 ) 0 CV (T2 T1) 0 0 ln 0 1 2 1 2 V V R T T ln 0 1 2 2 1 V V R T T Q2 Q1 0 1 1 2 2 T Q T Q 4 3 1 2 V V V V
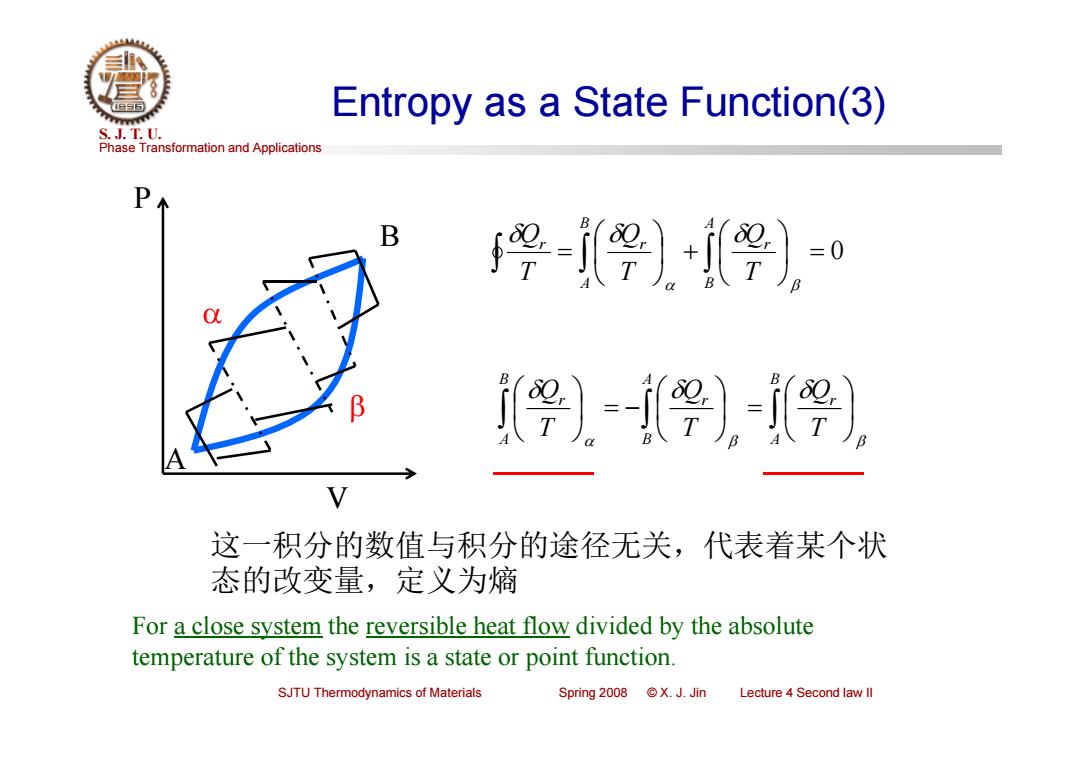
Entropy as a State Function(3) S.J.T.0. Phase Transformation and Applications -))= i〔婴)-婴-i婴) 这一积分的数值与积分的途径无关,代表着某个状 态的改变量,定义为熵 For a close system the reversible heat flow divided by the absolute temperature of the system is a state or point function. SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Entropy as a State Function(3) 0 AB r BA r r TQ TQ TQ BA r AB r BA r TQ TQ TQ P V A B 这一积分的数值与积分的途径无关,代表着某个状 态的改变量,定义为熵 For a close system the reversible heat flow divided by the absolute temperature of the system is a state or point function

The Second Law S.J.T.0. Phase Transformation and Applications The First Law:the conservation of energy and energy transfer in terms of heat and work. Energy is a state function. The Second Law:ENTROPY. Overview Heat engines,devices that convert thermal energy (heat)into mechanical energy (work). The first law places no limits on the amounts that can be converted. The second law is concerned with limits on the conversion of"heat"into s“work”by heat engines. 1824,a French engineer,Sadi Carnot,idealized heat engine. P.W.Atkins,The Second Law,Scientific American Books,1984 SJTU Thermodynamics of Materials Spring2o08©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II The Second Law The First Law: the conservation of energy and energy transfer in terms of heat and work. Energy is a state function. The Second Law: ENTROPY. Overview Heat engines, devices that convert thermal energy (heat) into mechanical energy (work). The first law places no limits on the amounts that can be converted. The second law is concerned with limits on the conversion of “heat” into “work” by heat engines. 1824, a French engineer, Sadi Carnot, idealized heat engine. P. W. Atkins, The Second Law, Scientific American Books, 1984

Measuring the entropy S.J.T.0. Phase Transformation and Applications Display MpIs网 K-1 Entropy Thermometer Monitor of heater SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Measuring the entropy