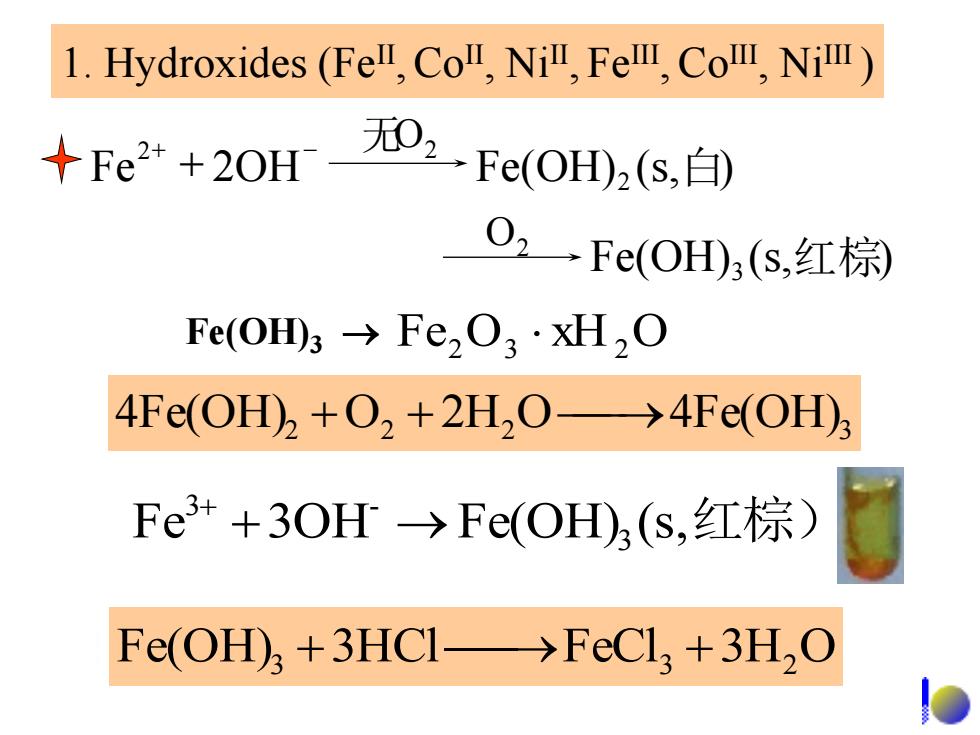
l.Hydroxides(Fel,Co,Ni,FeⅢ,CoⅢ,Ni) +Fe+2OH无和,-Fe(OH,s,白) O,→Fe(OH,(s,红棕 Fe(OHm3→Fe,03·xH,O 4Fe(OH)+O2+2H2O->4Fe(OH) Fe3++3OH→Fe(OH)h(s,红棕) Fe(OH+3HCI->FeCl,+3H,O
1. Hydroxides (FeII ,CoII, NiII , FeIII , CoIII, NiIII ) Fe2 O3 xH2 O 4Fe(OH)2 O2 2H2 O 4Fe(OH)3 Fe 2OH Fe(OH) (s,白) 2 2 无O2 - Fe(OH) (s,红棕 ) 3 O2 Fe3 3OH- Fe(OH)3 (s,红棕) Fe(OH)3 3HCl FeCl3 3H2 O Fe(OH)3
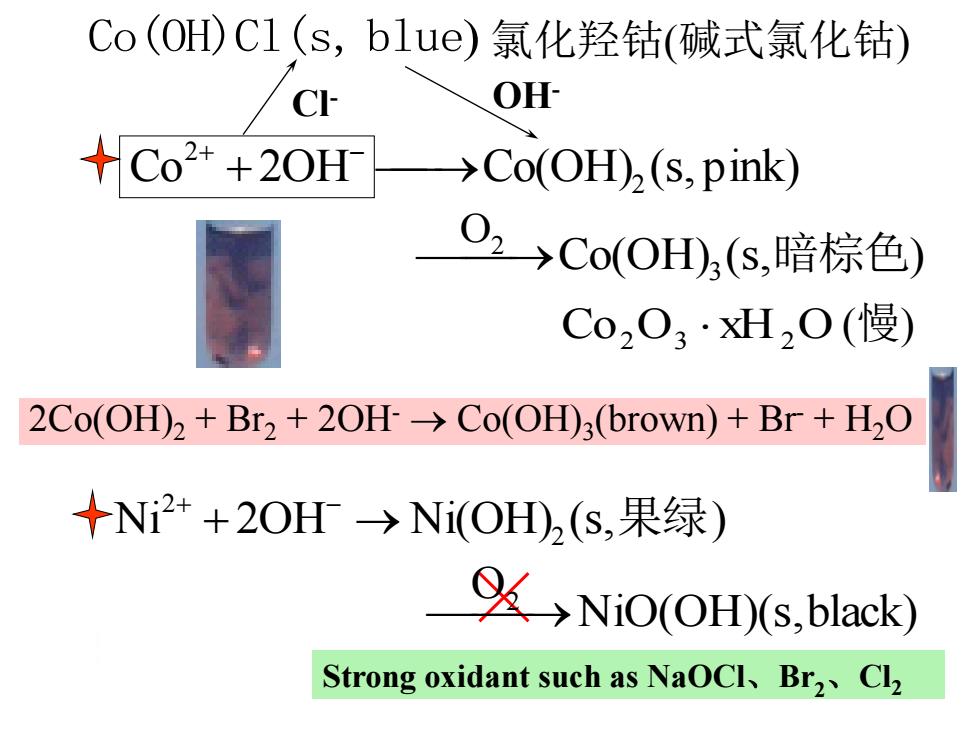
Co(OH)C1(s,blue)氯化羟钴(碱式氯化钴) C OH Co2++20H>Co(OH)(s,pink) O,→CoOH,s,暗棕色) Co2O3·2O(慢) 2Co(OH)2+Br2+20H>Co(OH)3(brown)+Br+H2O +N2++2OH→Ni(OH2(s,果绿) NiO(OH)(s.black) Strong oxidant such as NaOCl、Br2、Cl2
Co 2OH Co(OH) (s, pink) 2 2 - Co(OH)Cl(s, blue) 氯化羟钴(碱式氯化钴) Co2 O3 xH2 O (慢) Ni 2OH Ni(OH) (s, 绿 ) 2 2 - 果 Co(OH) (s, ) 3 O 2 暗棕色 NiO(OH)(s,black) O 2 Strong oxidant such as NaOCl、Br2、Cl2 2Co(OH)2 + Br2 + 2OH- Co(OH)3 (brown) + Br- + H2O Cl- OH-
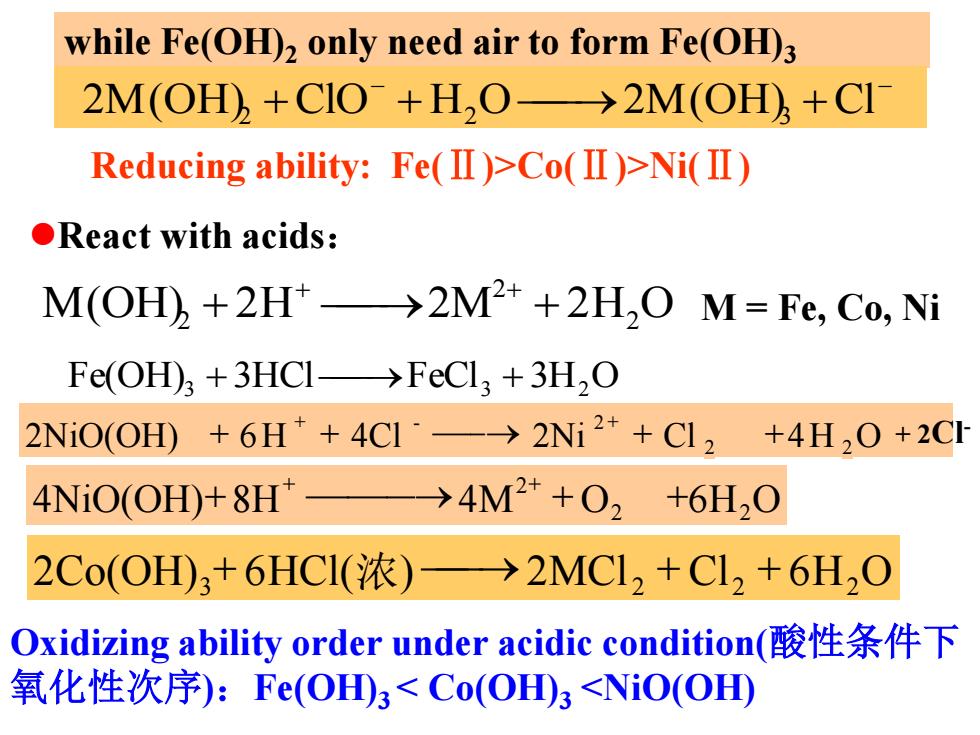
while Fe(OH)2 only need air to form Fe(OH)3 2M(OH)+CIO+H,O->2M(OH)+CI Reducing ability:Fe(Ⅱ)>Co(Ⅱ)>Ni(I) ●React with acids: M(OH)+2H>2M2+2H,O M=Fe,Co,Ni Fe(OH)+3HCI->FeCl3 +3H,O 2NiO(OHD+6H++4C1·-→2Ni2++C12 +4H,0+2C1 4NiO(OH)+8H" →4M2++02+6H20 2Co(OH3+6HCI(浓)→2MC1,+C1,+6H,O Oxidizing ability order under acidic condition(酸性条件下 氧化性次序):Fε(OH3<Co(OH)3<NiO(OH)
React with acids: M(OH) 2H 2M 2H2 O 2 2 M = Fe, Co, Ni Fe(OH)3 3HCl FeCl3 3H2 O Oxidizing ability order under acidic condition(酸性条件下 氧化性次序):Fe(OH)3 < Co(OH)3 <NiO(OH) while Fe(OH)2 only need air to form Fe(OH)3 2M(OH) 6HCl( ) 2MCl Cl 6H O 2M(OH) ClO H O 2M(OH) Cl 3 2 2 2 2 2 3 - - Reducing ability: 浓 Fe(Ⅱ)>Co(Ⅱ)>Ni(Ⅱ) 2Co(OH)3 6HCl(浓) 2MCl 2 Cl2 6H2O 4NiO(OH) 8H 4M O2 6H2O H2SO4 2 2NiO(OH) 6H 4Cl 2Ni Cl 2 4H 2O - 2 + 2Cl-

●Coordinating reaction(配位反应): M(OH)+2NH3->[M(NH)](M=Co,Ni) Fe(OH)2 +NH3
2 2 3 3 6 M(OH) 2NH [M(NH ) ] (M=Co,Ni) Coordinating reaction(配位反应): Fe(OH)2 + NH3
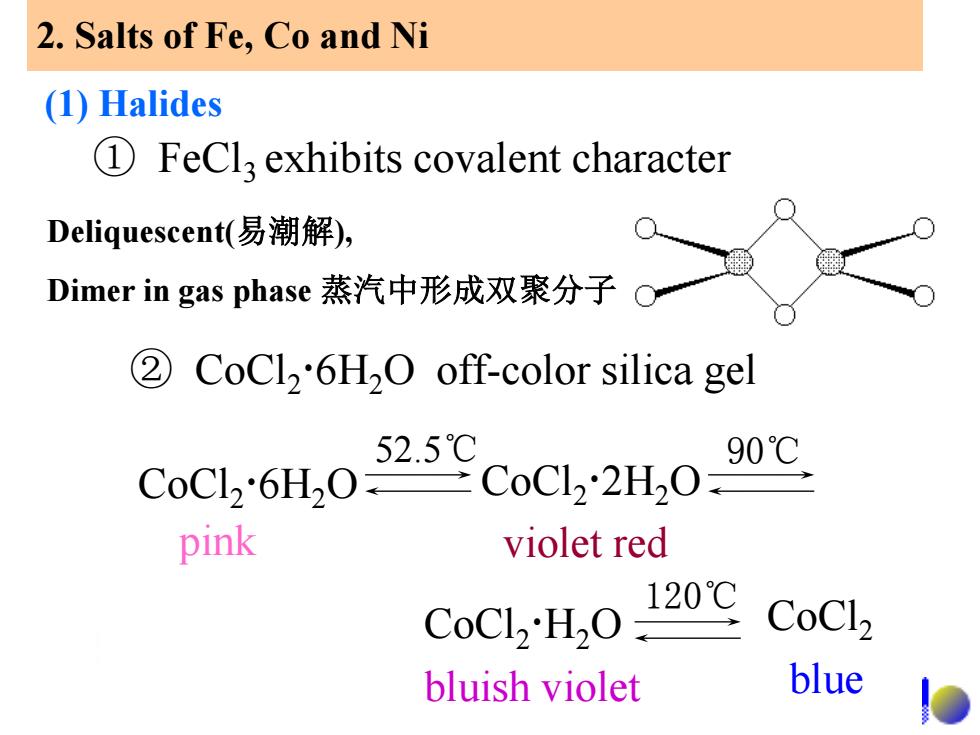
2.Salts of Fe,Co and Ni (1)Halides 1 FeCla exhibits covalent character Deliquescent(易潮解), Dimer in gas phase蒸汽中形成双聚分子 2 CoCl2'6H2O off-color silica gel 52.5℃ 90℃ CoCl2"6H2OCoCl2H,O pink violet red CoCl,'H2O 120℃ CoCl2 bluish violet blue
① FeCl3 exhibits covalent character Deliquescent(易潮解), Dimer in gas phase 蒸汽中形成双聚分子 ② CoCl2 6H2O off-color silica gel CoCl2 6H2O CoCl2 2H2O violet red CoCl2 H2O bluish violet pink 52.5℃ 90℃ 120℃ CoCl2 blue 2. Salts of Fe, Co and Ni (1) Halides