
上游充通大¥ e SHANGHAI JIAO TONG UNIVERSITY Chapter 9.Phase Diagram Il Immiscibility:不溶性 Spinodal Points:拐点 Simple Eutectic Diagram:简单共晶相图 Peritectic Phase diagram:包晶相图 Compounds:化合物 Conclusion Remarks Examples ONG UNN
Chapter 9. Phase Diagram II Immiscibility: 不溶性 Spinodal Points:拐点 Simple Eutectic Diagram: 简单共晶相图 Peritectic Phase diagram:包晶相图 Compounds:化合物 Conclusion Remarks Examples
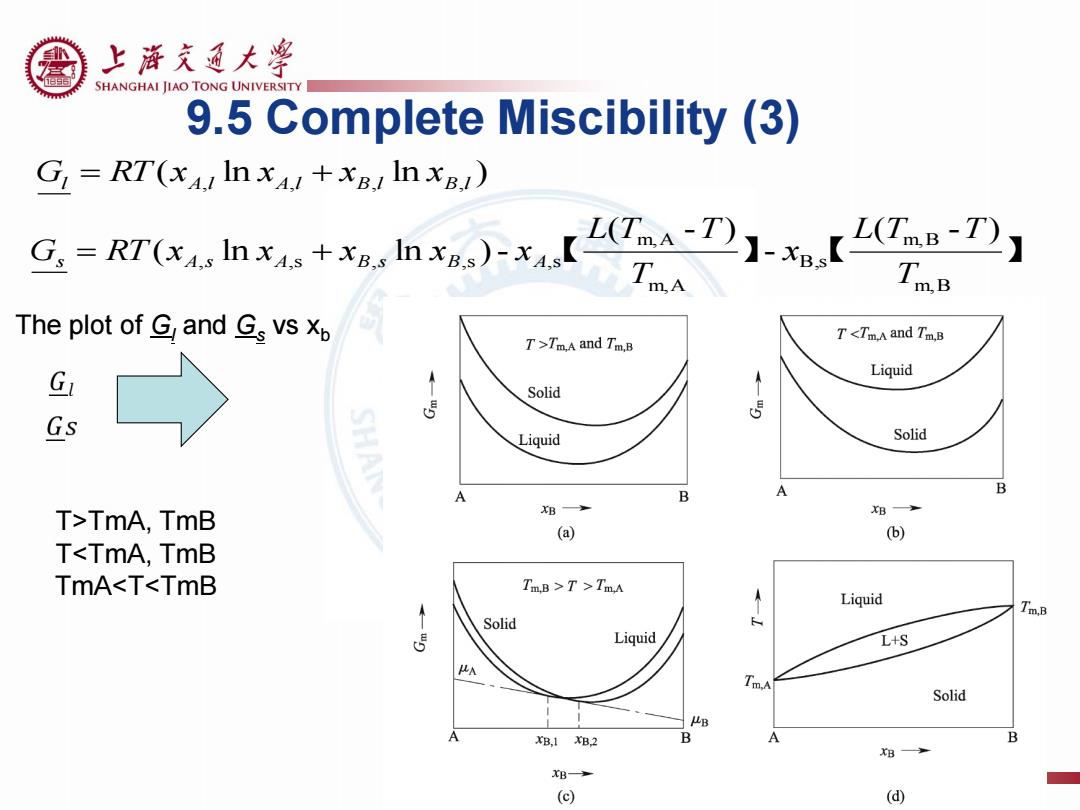
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY 9.5 Complete Miscibility (3) G RT(xAI In xA+xB In xB) uhe+.lh)-x【O】-【 wB The plot of G and Gs vs p T <TmA and Tm.B T>Tm.A and Tm.B G Liquid T Solid 5 5 Gs Liquid Solid B A B T>TmA,TmB B B (a) (b) T<TmA,TmB TmA<T<TmB TmB >T>TmA Liquid Tm.B Solid Liquid L+S A Solid B B,1B2 B A B B B (⊙ (d)
【 】 【 】 m,B m,B B ,s m,A m,A , ,s , ,s ,s ( - ) - ( - ) ( ln ln )- T L T T x T L T T G RT x x x x x s A s A B s B A ( ln ln ) l A,l A,l B,l B,l G RT x x x x 9.5 Complete Miscibility (3) The plot of Gl and Gs vs xb T>TmA, TmB T<TmA, TmB TmA<T<TmB
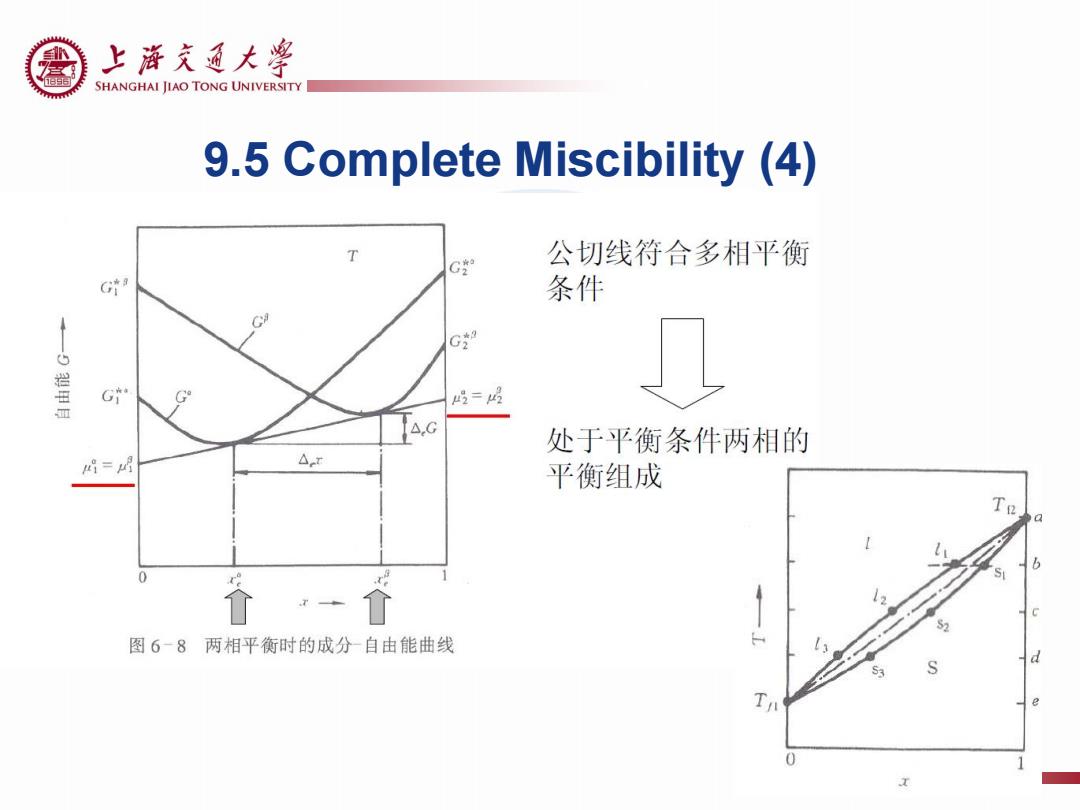
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY 9.5 Complete Miscibility (4) T C° 公切线符合多相平衡 G 条件 C G” G G =喝 处于平衡条件两相的 店= △r 平衡组成 a 图6-8两相平衡时的成分自由能曲线 d Ta
9.5 Complete Miscibility (4)
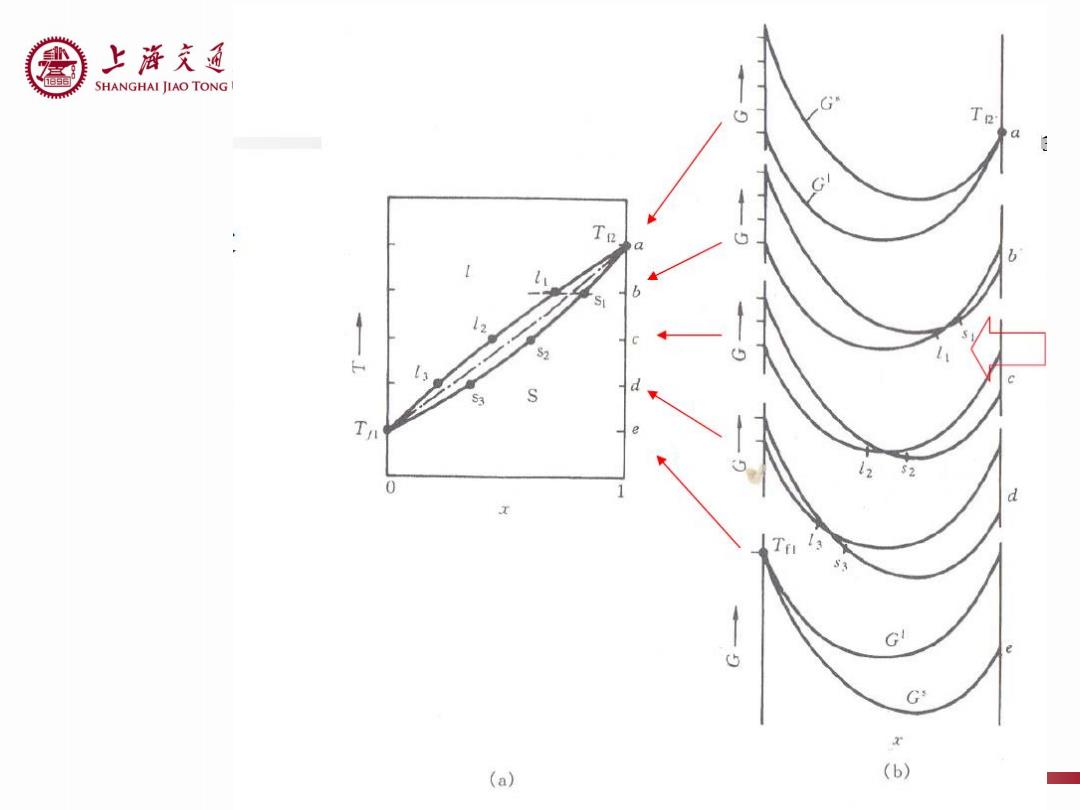
上游充通 SHANGHAI JIAO TONG G Te Te 0 d 1 G 5 G (a) (b)
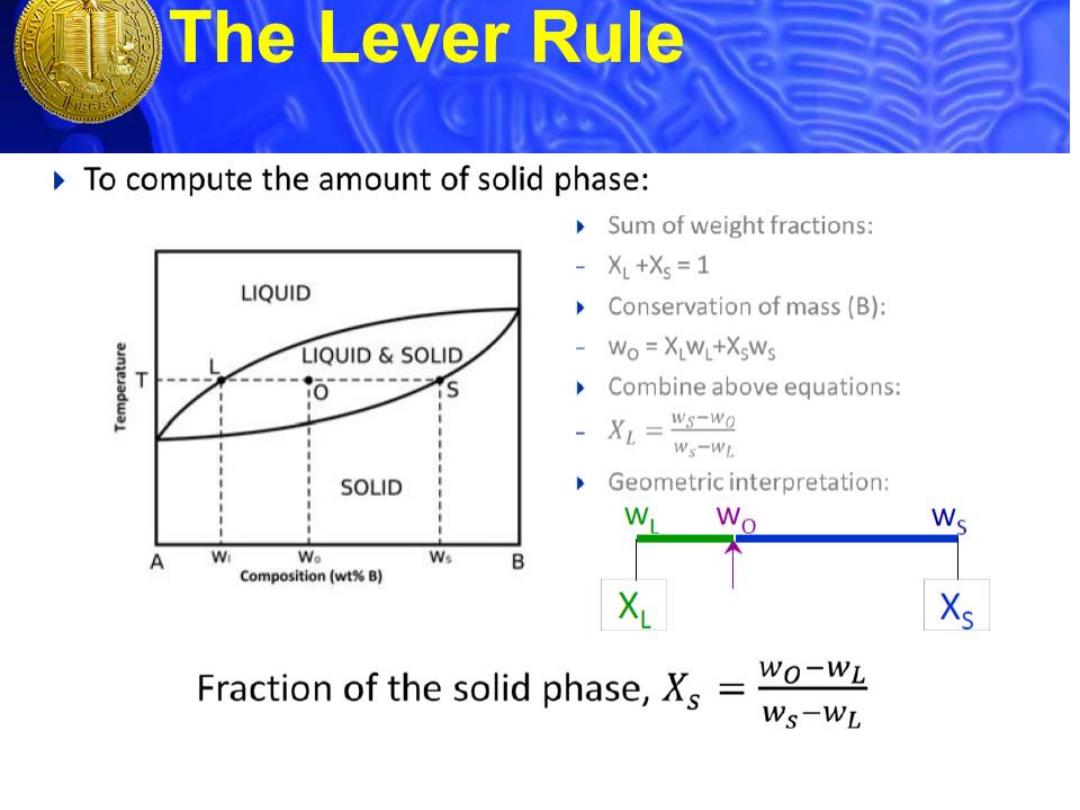
The Lever Rule To compute the amount of solid phase: Sum of weight fractions: XL+Xs =1 LIQUID Conservation of mass(B): LIQUID SOLID Wo XLWL+XsWs T Combine above equations: XL= ws-wo Ws-WL SOLID Geometric interpretation: W Wo Ws W Wo Ws B Composition(wt%B) X Fraction of the solid phase,Xs= Wo-WL Ws-WL