
Chemical properties: ①unstable 2HNO2-H2O+N2O3-H2O+NO+NO2 (blue) ②weak acid HNO2-H+NO2 NO+NO2 K9=6.0X104 nitrous acid (HNO2)
nitrous acid (HNO 2 ) NO+NO 2 Chemical properties : ② weak acid (blue ) ① unstable 2 2 _ HNO H + NO + 4 _ K a = 6.0 ×10 2HNO 2 H 2 O N 2 O 3 H 2 O NO NO 2 + + +

·Nitrite(亚硝酸盐) Preparation:treating the acidic oxides with a base NO,+NO+NaOH>2NaNO +H,O Properties: 1 Most nitrites are colorless and soluble in water (except for AgNO2) ②redox properties (E(HNO/NO)=1.04V) 2NO +2I+4H"2NO+I2 +2H,O 主 NO;+Fe2*+2H*>NO+Fe*+H,O 5NO;+2MnO;+6H>5NO;+2Mn2*+3H,O 3 metals having low activity correspond to nitrites of low thermal stability金属活泼性差,对应亚硝酸盐稳定性差 AgNO2<NaNO2(Mt的极化力)
• Nitrite (亚硝酸盐) Preparation: treating the acidic oxides with a base NO2 + NO+ NaOH⎯⎯→2NaNO2 + H2O Properties: ① Most nitrites are colorless and soluble in water (except for AgNO2) ③ metals having low activity correspond to nitrites of low thermal stability 金属活泼性差,对应亚硝酸盐稳定性差 主 AgNO2<NaNO2 (M+的极化力 ) ( (HNO /NO) 1.04V) A 2 E = 2NO 2I 4H 2NO I2 2H2O - - 2 + + + + + NO Fe 2H NO Fe H2O - 2 3 2 + + + + + + + 5NO 2MnO 6H 5NO 2Mn 3H2O - 2 3 -4 -2 + + + + + + ② redox properties
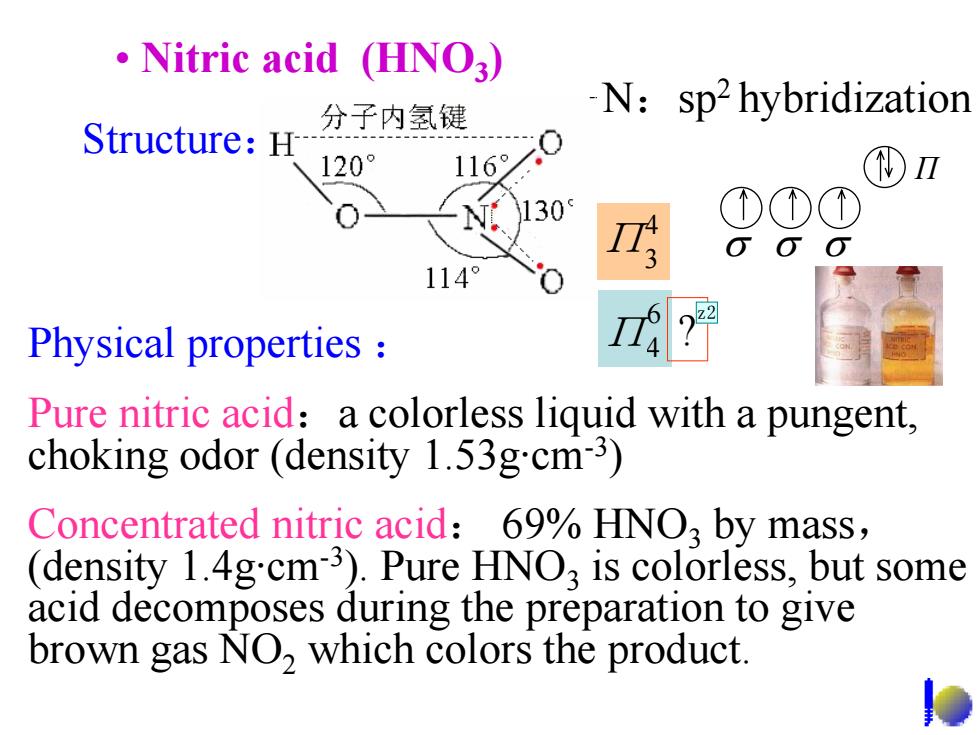
Nitric acid (HNO3) 分子内氢键 N:sp2hybridization Structure:H 120° 116° )Ⅱ N. 130 ①①① 114° Physical properties Pure nitric acid:a colorless liquid with a pungent, choking odor (density 1.53g.cm-3) Concentrated nitric acid:69%HNO3 by mass, (density 1.4g.cm3).Pure HNO,is colorless,but some acid decomposes during the preparation to give brown gas NO,which colors the product
• Nitric acid (HNO3) Physical properties : Pure nitric acid:a colorless liquid with a pungent, choking odor (density 1.53g·cm-3) Concentrated nitric acid: 69% HNO3 by mass, (density 1.4g·cm-3). Pure HNO3 is colorless, but some acid decomposes during the preparation to give brown gas NO2 which colors the product. 4 Π3 Structure: N:sp2 hybridization σ σ σ Π 6 Π4 ?z2

Chemical properties of HNO3 1 Powerful oxidizing agent HNO,+非金属单质→相应高价酸+NO 4HN03+3C→3C02(g)+4NO(g)+2H20 5HNO3 +3P+2H2O->3H,PO+5NO(g) 2HNO3+S→H,SO4+2NO 10HNO3+3I2>6HIO3+10N0+2H2O
Chemical properties of HNO 3 ① Powerful oxidizing agent HNO 3 +非金属单质 ⎯⎯→相应高价酸 + NO 10HNO 3 + 3I 2 ⎯⎯→6HIO 3 +10NO + 2H 2 O 2HNO 3 + S ⎯⎯→ H 2SO 4 + 2NO 5HNO 3P 2H O 3H PO 5NO(g) 3 + + 2 ⎯⎯→ 3 4 + 4HNO 3 + 3C ⎯⎯→3CO 2 (g) + 4NO(g) + 2H 2 O

Concentrated nitric acid will oxidize almost all metals,but the exact product depends on the metal (the reducing agent)and acid concentration. Cu+4HNO3(浓→Cu(NO3)2+2NO2+2H2O 3Cu+8HNO,(稀一3Cu(NO3)2+2NO+4H2O Au,Pt,Nb,Ta etc don't react with HNO3 concentrated nitric acid Copper reacts with
Concentrated nitric acid will oxidize almost all metals, but the exact product depends on the metal (the reducing agent) and acid concentration. Cu + 4HNO 3 (浓) Cu(NO 3 ) 2 + 2NO 2 + 2H 2 O 3Cu + 8HNO 3 (稀) 3Cu(NO 3 ) 2 + 2NO + 4H 2 O Copper reacts with concentrated nitric acid Au, Pt, Nb, Ta etc don’t react with HNO 3 z1