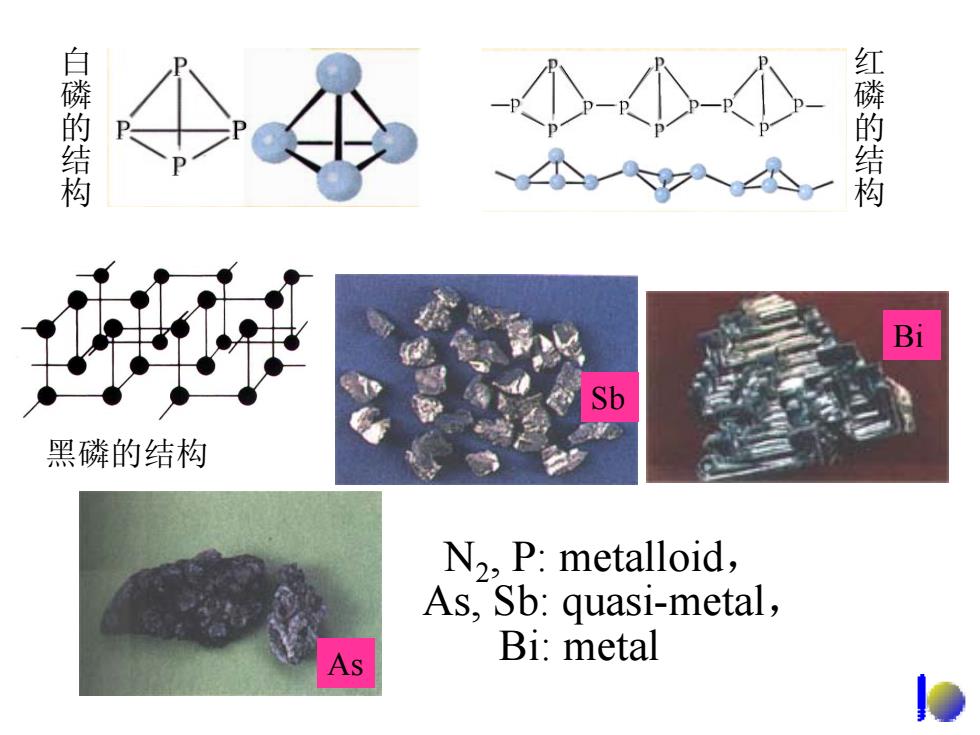
白磷的结构 红磷的结构 对 Bi Sb 黑磷的结构 N2,P:metalloid, As,Sb:quasi-metal, Bi:metal
As Sb Bi N2, P: metalloid, As, Sb: quasi-metal, Bi: metal 白磷的结构 红磷的结构 黑磷的结构
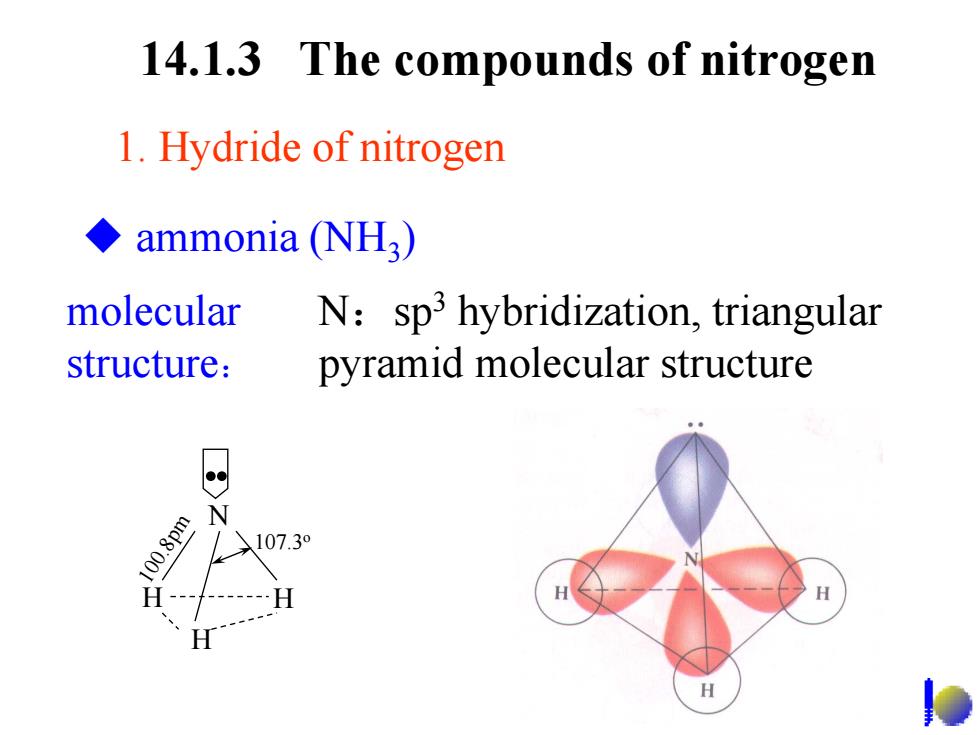
14.1.3 The compounds of nitrogen 1.Hydride of nitrogen ◆ammonia(NH3) molecular N:sp3 hybridization,triangular structure: pyramid molecular structure N 100.8pm 107.30
14.1.3 The compounds of nitrogen molecular structure: N :sp 3 hybridization, triangular pyramid molecular structure 1. Hydride of nitrogen N H 100.8pm 107.3 o .. H H ammonia (NH 3 )

Preparation: In laboratory: 2NHCI+Ca(OH),>CaCl,+2H2O+2NH3(g) 雨林木风1 In industry process: N2+3H2450-500C30MPae→2NH, Chemical properties: 1 Soluble in water forming monoprotic weak base NH+H,ONH·H,O-NH4+OH 2 Strong reducing ability 4NH3 +302(pure)->2N2+6H2O 4NH,+50,(ain0→4N0+61,0
In industry process: 2NH Cl Ca(OH) CaCl 2H O 2NH (g) 4 + 2 ⎯⎯→ 2 + 2 + 3 N 2 3H 2 2NH 3 ⎯ 450 ⎯ → ⎯~500 ⎯⎯C 30MPa ⎯⎯⎯⎯ Fe + ° Preparation : In laboratory : Chemical properties : ① Soluble in water forming monoprotic weak base ② Strong reducing ability _ NH3 + H 2 O NH3 ⋅H 2 O NH4 +OH + 4NH 5O (air) 4NO 6H O 4NH 3O (pure) 2N 6H O 2 Pt 3 2 3 2 2 2 + ⎯⎯→ + + → + 800 o c 雨林木风1
3 coordination reaction Ht+NH3→NH4 Ag+2NH3→[AgNH3)2]H 4Substitution reaction 2NH,+2Na- 70c→2NaNH2+H2 催化 ◆NH,-NH,联氨(肼),NH氏亚氨基,N<氮化物 hydrazine ◆NH,OH羟氦,Ammonium hydroxide
④ Substitution reaction + + + + + → + → Ag 2NH [Ag(NH ) ] H NH NH 3 3 2 3 4 2 2 570 C 2NH3 + 2Na ⎯⎯ →⎯ 2NaNH + H ° 催化 ③ coordination reaction NH2-NH2联氨(肼),NH 亚氨基,N 氮化物 hydrazine NH2OH 羟氨, Ammonium hydroxide
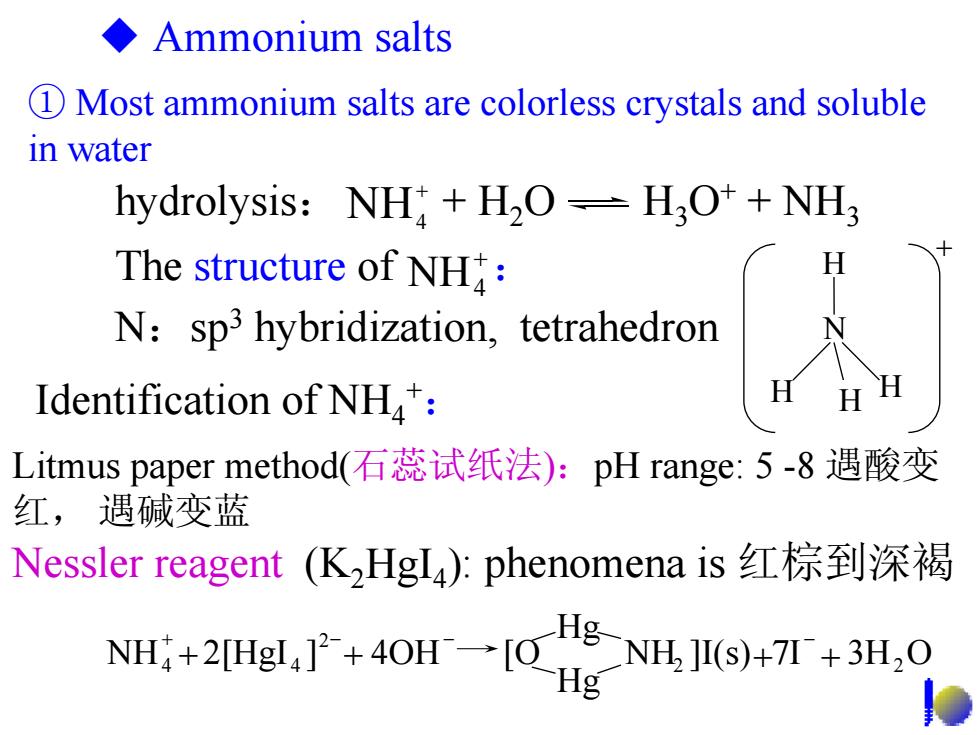
◆Ammonium salts D Most ammonium salts are colorless crystals and soluble in water hydrolysis:NH H2O-H3O++NH3 The structure of NH: N:sp3 hybridization,tetrahedron Identification of NH: Litmus paper method(石蕊试纸法):pH range:5-8遇酸变 红,遇碱变蓝 Nessler reagent(KHgI4):phenomena is红棕到深褐 NH4+2[Hgl4]2+4OH→[O g-NH.]I(s)+7+3H.O Hg
① Most ammonium salts are colorless crystals and soluble in water H N H H H + Ammonium salts Litmus paper method(石蕊试纸法):pH range: 5 -8 遇酸变 红, 遇碱变蓝 + NH4 The structure of : Nessler reagent (K2HgI4): phenomena is 红棕到深褐 N:sp3 hybridization, tetrahedron Identification of NH4+: NH ]I(s) 7I 3H O Hg Hg NH 2[HgI ] 4OH [O 2 2 2 4 + 4 + + + + − − − hydrolysis: + H2O H3O+ + NH3 + NH4