
上浒充通大粤 SHANGHAI JIAO TONG UNIVERSITY 9.1 Freezing point depression(2) L=Tm△Smelting 及是 →ASmelting=L/Tm L(T-T) Tm AGmelting =RTIn apme as.pure RTIn- L(T-T) T<Tm s.pure m &i,pure>1 The subcooled liquid is not stable,super active.The activity is larger than 1,the normal liquid 6
9.1 Freezing point depression (2) L = Tm ΔSmelting ΔSmelting = L/Tm m m . , . , melting m m melting ( - ) ln ln ( - ) (1 T L T T a a RT a a G RT T L T T T T G L s pure l pure s pure l pure m ) T<Tm αl,pure >1 The subcooled liquid is not stable, super active. The activity is larger than 1, the normal liquid 6

上浒充通大粤 e SHANGHAI JIAO TONG UNIVERSITY Consider the case where B is added into A to form a solution. Liquid:ideal solution Solid:immiscible T→x Equilibrium:Pure solid A and solution AB at T' Solid A Solution AB A XB→ B A:pure solid--solution Fig.9.2 Plot of the activity of Aliquid AO TONG at T<TmA versus composition. A:pure solid-=pure liquid = solution Melting Dissolving L(T-T) △G=RTln A.soluion To a1.pure 7
>1 Consider the case where B is added into A to form a solution. Liquid: ideal solution Solid: immiscible Equilibrium: Pure solid A and solution AB at T’ l pure l solution a a G RT . , ln Solid A Solution AB A: pure solidsolution A: pure solidpure liquid Melting m m melting ( - ) T L T T G 7 Dissolving solution
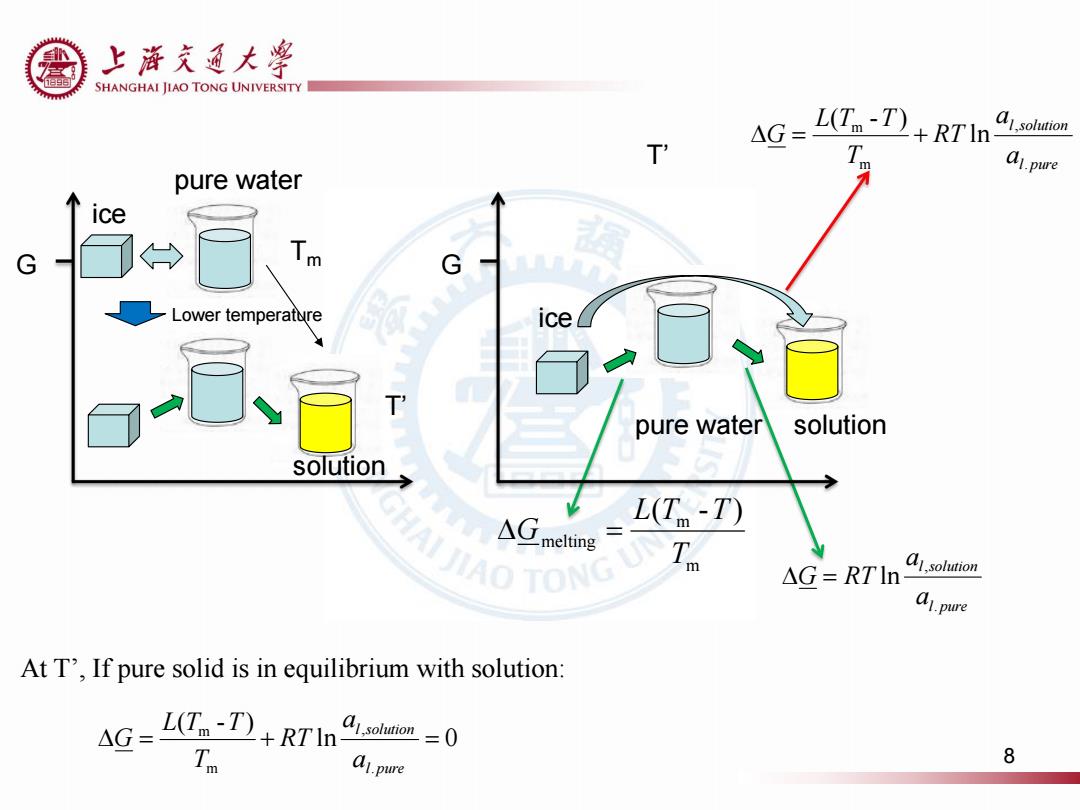
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY L(n-I)+RTI T 4G m a1.pure pure water ice G G Lower temperature ice pure water solution solution NAIJIAO TONG △G L(T-T) elting △G=RTln a1,solution a1.pure At T',If pure solid is in equilibrium with solution: AG=L(T-)+RTIn bon=0 A1.pure 8
T’ G ice ice pure water solution l pure l solution a a G RT . , ln m m melting ( - ) T L T T G G l pure l solution a a RT T L T T G . , m m ln ( - ) ln 0 ( - ) . , m m l pure l solution a a RT T L T T G At T’ , If pure solid is in equilibrium with solution: Tm pure water solution T’ Lower temperature 8
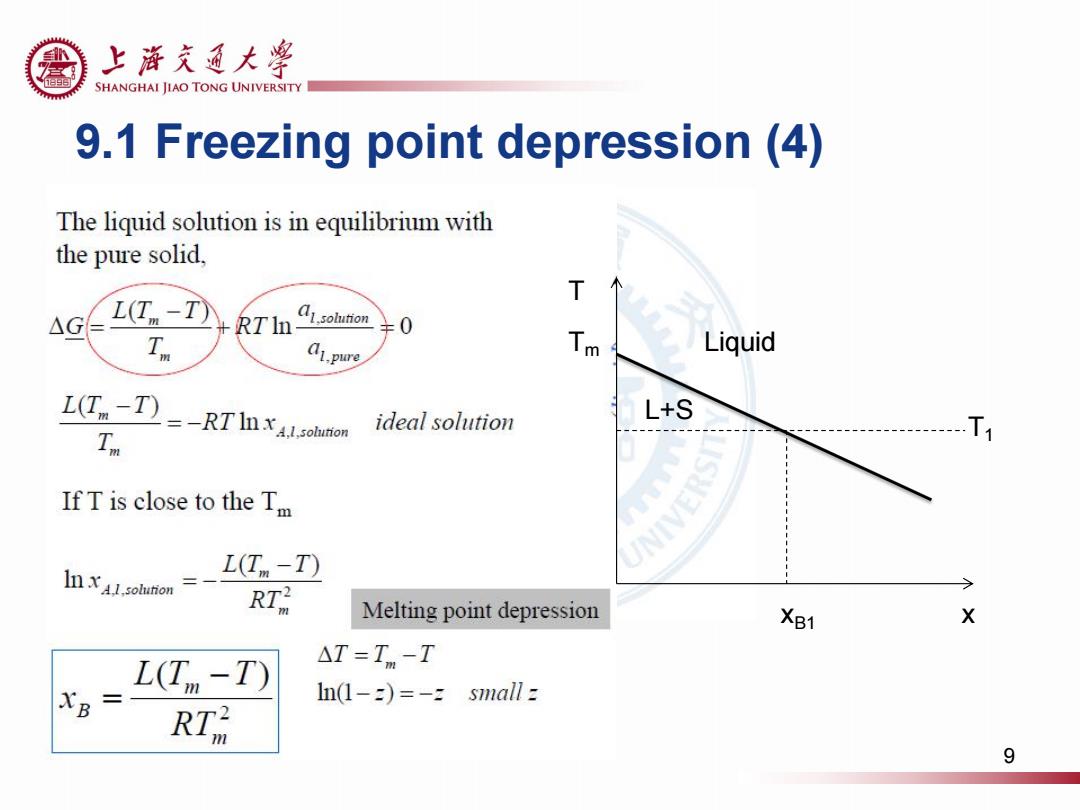
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY 9.1 Freezing point depression (4) The liquid solution is in equilibrium with the pure solid. T △G L(T-T RTIolunon T a1.pure 人 Liquid L(T-T )=-RT ohunon ideal solution L+S Tn If T is close to the Tm NIVE In x1.solution L(T-T) RT Melting point depression XB1 X L(T -T) △T=Tm-T XB 三 n1-2)=-zs7 nall z RT品 9
9.1 Freezing point depression (4) Tm T Liquid L+S T1 xB1 x 9