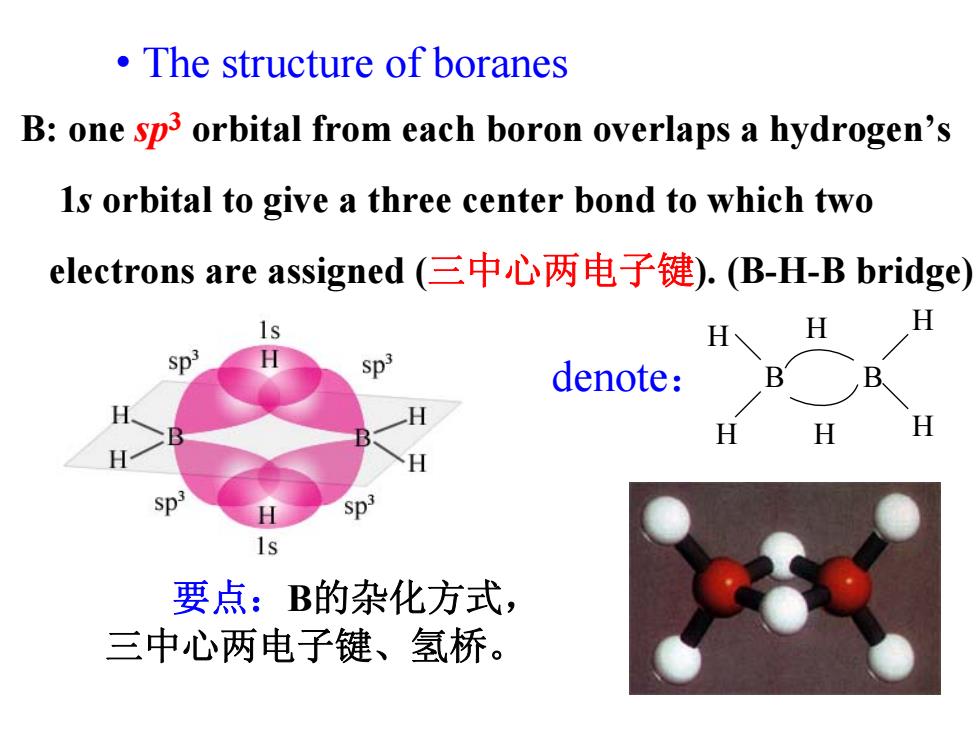
The structure of boranes B:one sp3 orbital from each boron overlaps a hydrogen's Is orbital to give a three center bond to which two electrons are assigned(三中心两电子键).(B-H-B bridge) H H H Sp3 denote: B B H H H Sp 1s 要点:B的杂化方式, 三中心两电子键、氢桥
• The structure of boranes B: one sp3 orbital from each boron overlaps a hydrogen’s 1s orbital to give a three center bond to which two electrons are assigned (三中心两电子键). (B-H-B bridge) denote: H H B B H H H H 要点:B的杂化方式, 三中心两电子键、氢桥
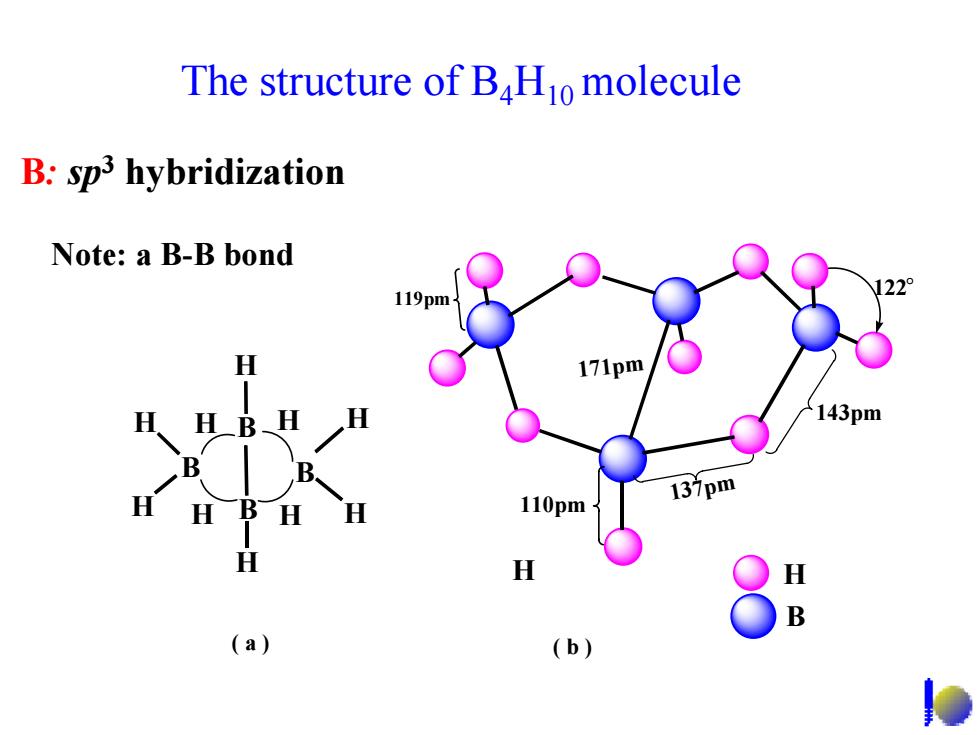
The structure of BHo molecule B:sp3 hybridization Note:a B-B bond 119pm H 143pm 137pm 110pm H B (a) (b)
The structure of B4H10 molecule 119pm 110pm 137pm 143pm 122。 171pm H B ( b ) B B B B H H H H H H H H H H H ( a ) B: sp3 hybridization Note: a B-B bond
The chemistry properties of boranes ①auto ignition(自燃) B2H6(g)+302(g)→B203(S)+3H2O(g) △H=-2034 kJ.mol high energy fuel,virulent(剧毒) with a green flame Borane burning ②hydrolysis B2H6(g)+3H2O(①)→2H3B03(S)+6H2(g) △H=-509.3 kJ.mol 水下火箭燃料
• The chemistry properties of boranes ① auto ignition (自燃) high energy fuel,virulent(剧毒) ② hydrolysis with a green flame Borane burning B H (g) 3O (g) B O (s) 3H O(g) 2 6 + 2 ⎯⎯→ 2 3 + 2 B H (g) 3H O(l) 2H BO (s) 6H (g) 2 6 + 2 ⎯⎯→ 3 3 + 2 △rHm = -509.3kJ⋅mol-1 水下火箭燃料 △rHm -1 = -2034kJ⋅mol
3 addition reaction(free orbit) B,H。+C0→2[HB←C0] B,H6+2NH3→BH2·(NH3)2]+[BH4] 2LiH+B2H6→2LiBH4 Good reducing agent 2NaH+B2H6→2NaBH4 ④chlorizated by Cl2 B,H6(g)+6C12(g)→2BC13(I)+6HC1 △H品=-1376 kJ.mol
③ addition reaction (free orbit) B H CO 2[H B CO] 2 6 + ⎯⎯→ 3 ← ④ chlorizated by Cl2 B H (g) 6Cl (g) 2BCl (l) 6HCl 2 6 + 2 ⎯⎯→ 3 + -1 △rHm = -1376kJ⋅mol 2NaH + B2H6 ⎯⎯→2NaBH4 2LiH + B2H6 ⎯⎯→2LiBH4 + − B H + 2NH ⎯⎯→[BH ⋅(NH ) ] +[BH ] 2 6 3 2 3 2 4 :C O: ⋅ ⋅ ⋅ ⋅ Good reducing agent
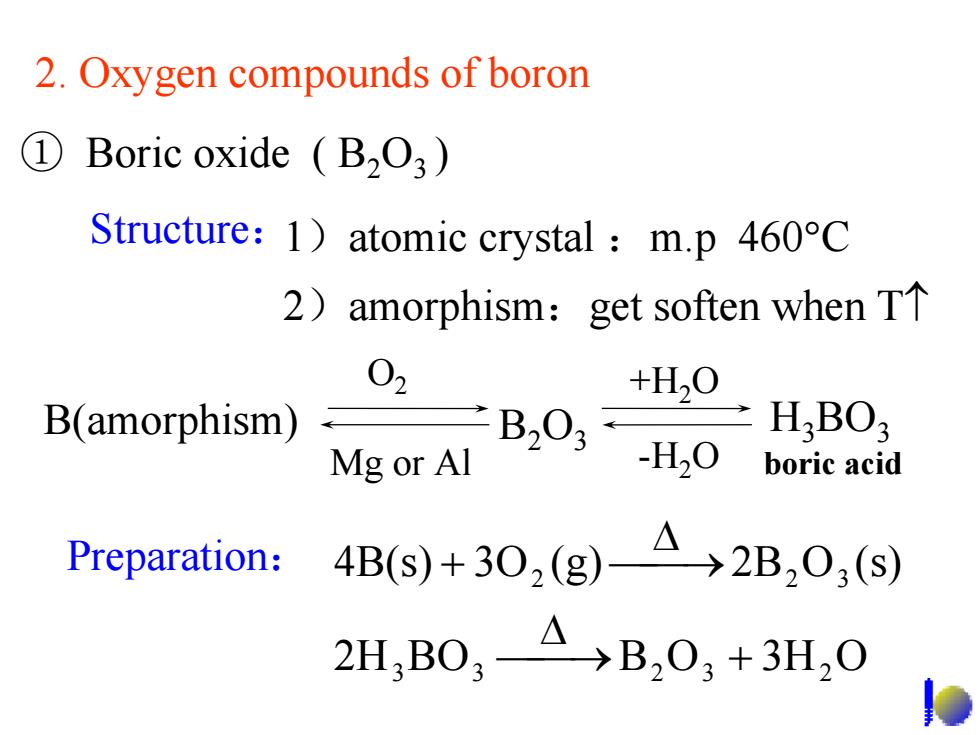
2.Oxygen compounds of boron ① Boric oxide (B2O3) Structure:1)atomic crystal m.p 460C 2)amorphism:get soften when TT 02 +H20 B(amorphism)- Mg or Al B203H,0 H.BO; boric acid Preparation: 4B(S+30,(g)4→2B,0,(s) 2H,B0,4)B,03+3H,0
① Boric oxide ( B2O3 ) O2 +H2O B(amorphism) B2O3 H3BO3 Mg or Al -H2O Preparation: 2H BO B O 3H O 4B(s) 3O (g) 2B O (s) 3 3 2 3 2 2 2 3 ⎯⎯→ + Δ ⎯⎯→ Δ + 1)atomic crystal :m.p 460°C 2)amorphism:get soften when T↑ 2. Oxygen compounds of boron Structure: boric acid