
The features of electron deficient compounds a.forming coordination compound HBF HF→BFg b.forming Al2Cl molecules in gas phase Al:ns2npl (sp3杂化) Cl:2s22p5
Cl Cl Al Cl Cl Cl Cl Al The features of electron deficient compounds b. forming Al2Cl6 molecules in gas phase HF BF3 a. forming coordination compound HBF4 Al:ns2np1 (sp3杂化) Cl:2s22p5
General properties of boron family elements ·Except for B which is a metalloid(非金属),Al, Ga,In,Tl are all metals. Oxidation numbers:B,Al,Ga:(+3) In:(+1,+3) T1:(+1) Maximum coordination numbers: B:4 example:HBF4(sp3杂化) others:6 example:Na3AlF6(sp3d杂化)
General properties of boron family elements • Except for B which is a metalloid(非金属), Al , Ga ,In ,Tl are all metals. • Oxidation numbers : B ,Al ,Ga :(+3) In :(+1 ,+3) Tl :(+1) • Maximum coordination numbers : B :4 example :HBF4 (sp 3杂化) others :6 example :Na 3AlF6 (sp 3 d 2杂化 )
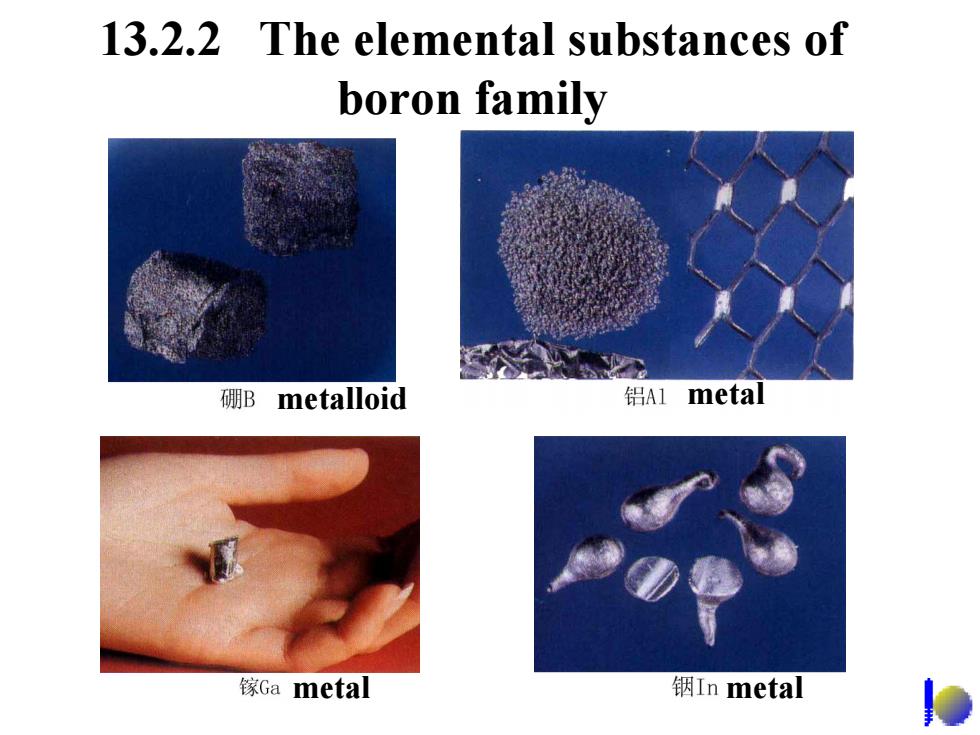
13.2.2 The elemental substances of boron family 硼B metalloid 铝Al metal 镓Ga metal 铟In metal
13.2.2 The elemental substances of boron family metalloid metal metal metal
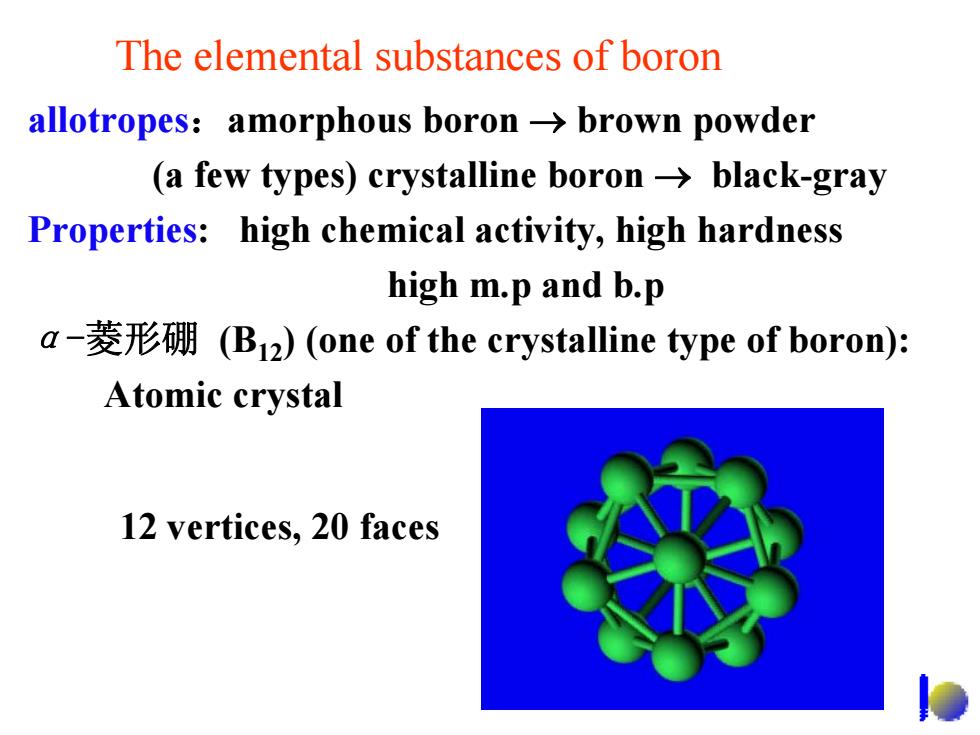
The elemental substances of boron allotropes:amorphous boron->brown powder (a few types)crystalline boron->black-gray Properties:high chemical activity,high hardness high m.p and b.p a-菱形硼(B12)(one of the crystalline type of boron): Atomic crystal 12 vertices,20 faces
The elemental substances of boron allotropes :amorphous boron → brown powder (a few types) crystalline boron → black-gray Properties: high chemical activity, high hardness high m.p and b.p α-菱形硼 (B12) (one of the crystalline type of boron): Atomic crystal 12 vertices, 20 faces
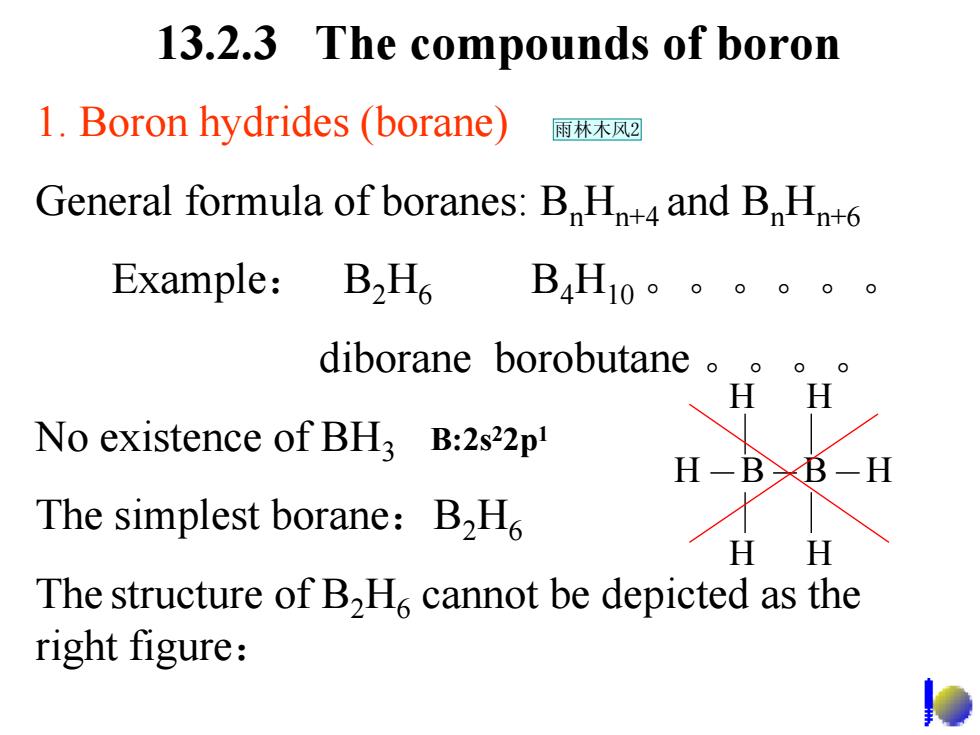
13.2.3 The compounds of boron 1.Boron hydrides (borane) 雨林木风2 General formula of boranes:B Ht4 and BHn+6 Example:B2H6B4Hio。。。。。。 diborane borobutane H H No existence of BHz B:2s22p H-BXB-H The simplest borane:B2H HH The structure of B,H.cannot be depicted as the right figure:
13.2.3 The compounds of boron 1. Boron hydrides (borane) General formula of boranes: B n Hn+4 and B n Hn+6 Example : B 2 H 6 B 4 H10。。。。。。 diborane borobutane 。。。。 No existence of BH 3 The simplest borane : B 2 H 6 The structure of B 2 H 6 cannot be depicted as the right figure : H H B B H H H H B:2s 22p 1 雨林木风2