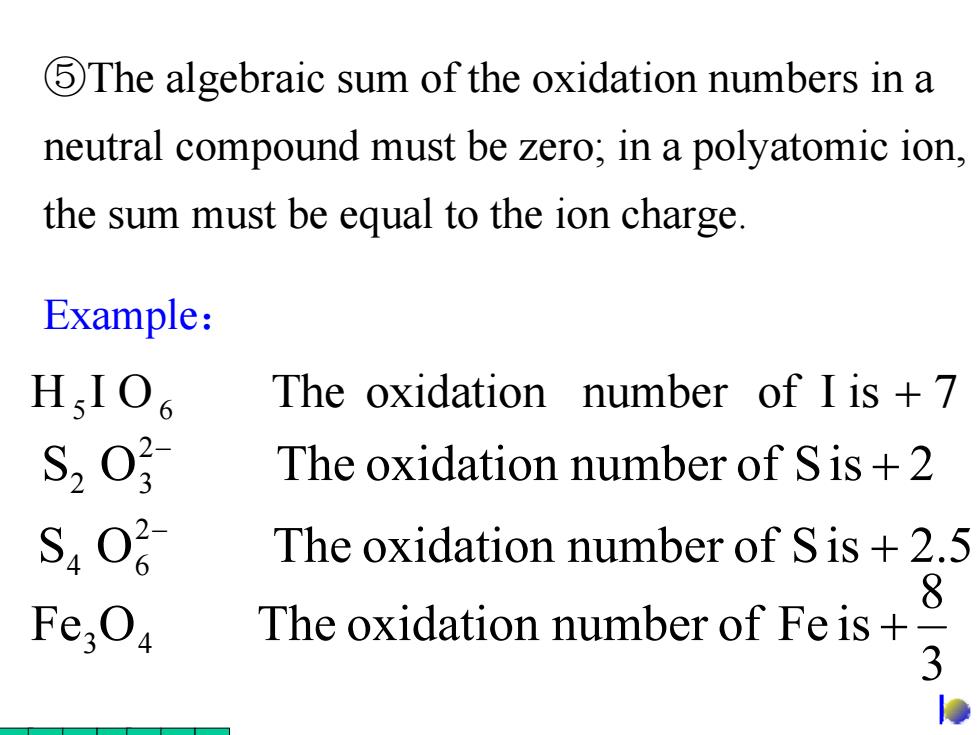
5The algebraic sum of the oxidation numbers in a neutral compound must be zero;in a polyatomic ion, the sum must be equal to the ion charge. Example: HIO The oxidation number of I is +7 S20 The oxidation number of Sis +2 S40 The oxidation number of Sis +2.5 8 Fe,Oa The oxidation number of Fe is+ 3 lo
Example: ⑤The algebraic sum of the oxidation numbers in a neutral compound must be zero; in a polyatomic ion, the sum must be equal to the ion charge. H I O The oxidation number of I is 7 5 6 + 3 8 Fe O The oxidation number of Fe is 3 4 + S O The oxidation number of Sis 2.5 2 4 6 + − S O The oxidation number of Sis 2 2 2 3 + −
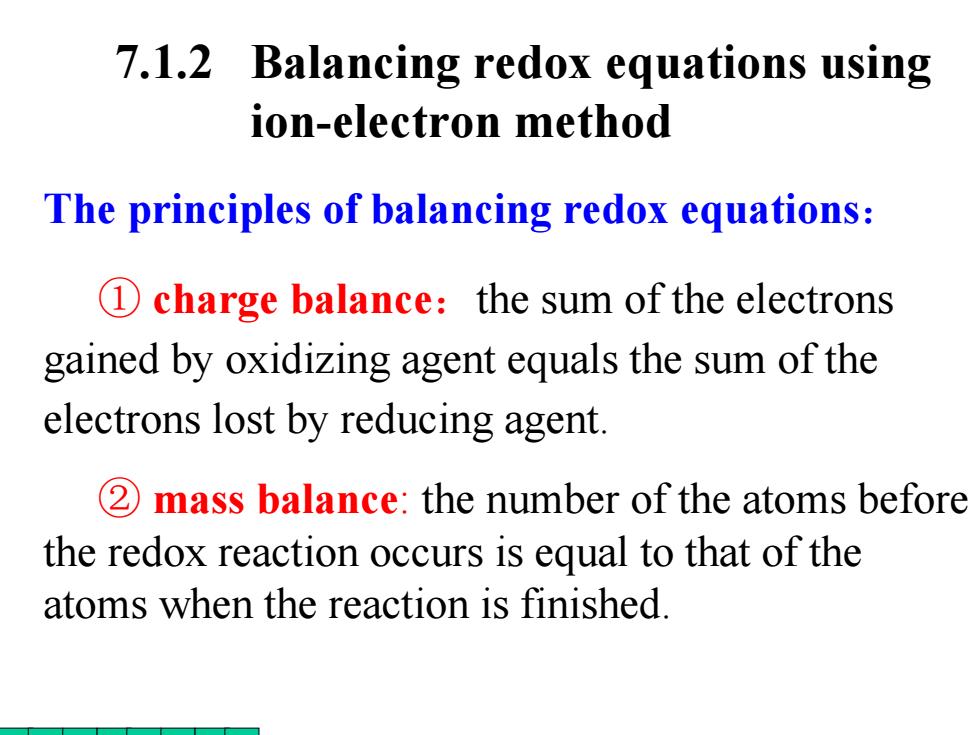
7.1.2 Balancing redox equations using ion-electron method The principles of balancing redox equations: 1 charge balance:the sum of the electrons gained by oxidizing agent equals the sum of the electrons lost by reducing agent. 2 mass balance:the number of the atoms before the redox reaction occurs is equal to that of the atoms when the reaction is finished
The principles of balancing redox equations : ① charge balance :the sum of the electrons gained by oxidizing agent equals the sum of the electrons lost by reducing agent. ② mass balance: the number of the atoms before the redox reaction occurs is equal to that of the atoms when the reaction is finished. 7.1.2 Balancing redox equations using ion-electron method

The general approach to balance a redox equation: 1 write out the unbalanced reaction equation in ionic form(note:gas,pure liquid,solid and weak electrolyte must be written out in molecular form):用离子式写出主要反应 物和产物(气体、纯液体、固体和弱电解质则写分子式)。 2 separate the equation into two half-reactions:the oxidation one and reduction one分别写出氧化剂被还原和还原剂 被氧化的半反应。 3 balance each half-reaction for number and type of atoms and charges分别配平两个半反应方程式,等号两边的各种元素的 原子总数各自相等且电荷数相等
The general approach to balance a redox equation : ① write out the unbalanced reaction equation in ionic form (note: gas, pure liquid, solid and weak electrolyte must be written out in molecular form): 用离子式写出主要反应 物和产物 (气体、纯液体、固体和弱电解质则写分子式 ) 。 ② separate the equation into two half-reactions: the oxidation one and reduction one 分别写出氧化剂被还原和还原剂 被氧化的半反应 。 ③ balance each half-reaction for number and type of atoms and charges 分别配平两个半反应方程式,等号两边的各种元素的 原子总数各自相等且电荷数相等

4If the oxidation and reduction half-reactions contain different numbers of electrons,we need to multiply one or both half-reactions to equal the number of electrons,then add the two half-reactions together and balance the final equation by inspection. 确定两半反应方程式得、失电子数目的最小公倍数。将两个半反应方程式中各 项分别乘以相应的系数,使得、失电子数目相同。然后,将两者合并,就得到 了配平的氧化还原反应的离子方程式。有时根据需要可将其改为分子方程式。 Example1-1:Balance the following reaction equation KMnO4(aq)+KzS03(aq)TMnS04(aq)+K2S04(aq)
Example1-1:Balance the following reaction equation ④If the oxidation and reduction half-reactions contain different numbers of electrons, we need to multiply one or both half-reactions to equal the number of electrons, then add the two half-reactions together and balance the final equation by inspection. 确定两半反应方程式得、失电子数目的最小公倍数。将两个半反应方程式中各 项分别乘以相应的系数,使得、失电子数目相同。然后,将两者合并,就得到 了配平的氧化还原反应的离子方程式。有时根据需要可将其改为分子方程式。 KMnO4 (aq) + K2SO3 (aq) → MnSO4 (aq) + K2SO4(aq) H+
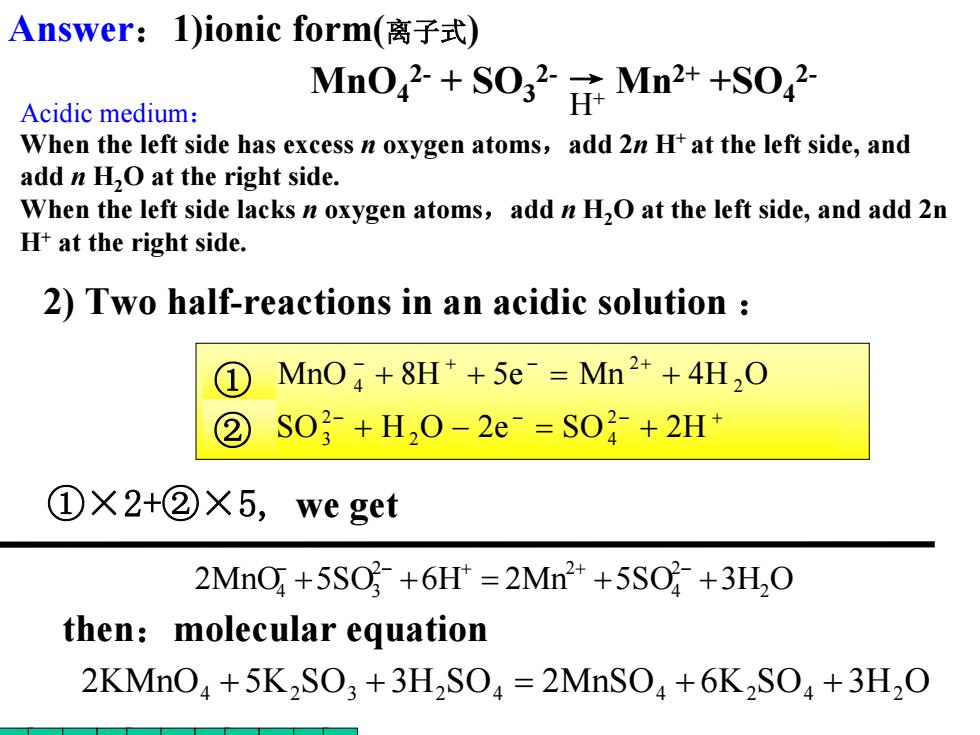
Answer:1)ionic form(离子式) Acidic medium: MnO+O Mn2++SO When the left side has excess n oxygen atoms,add 2n H+at the left side,and add n H2O at the right side. When the left side lacks n oxygen atoms,add n H.O at the left side,and add 2n H+at the right side. 2)Two half-reactions in an acidic solution ① MnO+8H*+5e Mn2++4H,O ② S0}+H,0-2e=S0?+2H* ①×2+②X5,we get 2MnO +5S0+6H*=2Mn2*+5SO+3H,O then:molecular equation 2KMnO+5K2SO3 +3H2SO=2MnSO+6K2SO+3H2O
− − − + − + − + + − = + + + = + SO H O 2e SO 2H MnO 8H 5e Mn 4H O 2 2 4 2 3 2 2 ① 4 ② ①×2+②×5, we get 2MnO 5SO 6H 2Mn 5SO 3H2O 24 2 2 4 + 3 + = + + − − + + − 2) Two half-reactions in an acidic solution : 2KMnO4 + 5K2SO3 + 3H2SO4 = 2MnSO4 + 6K2SO4 + 3H2O then:molecular equation MnO42- + SO32- → Mn2+ +SO42- H+ Answer:1)ionic form(离子式) Acidic medium: When the left side has excess n oxygen atoms,add 2n H+ at the left side, and add n H2O at the right side. When the left side lacks n oxygen atoms,add n H2O at the left side, and add 2n H+ at the right side