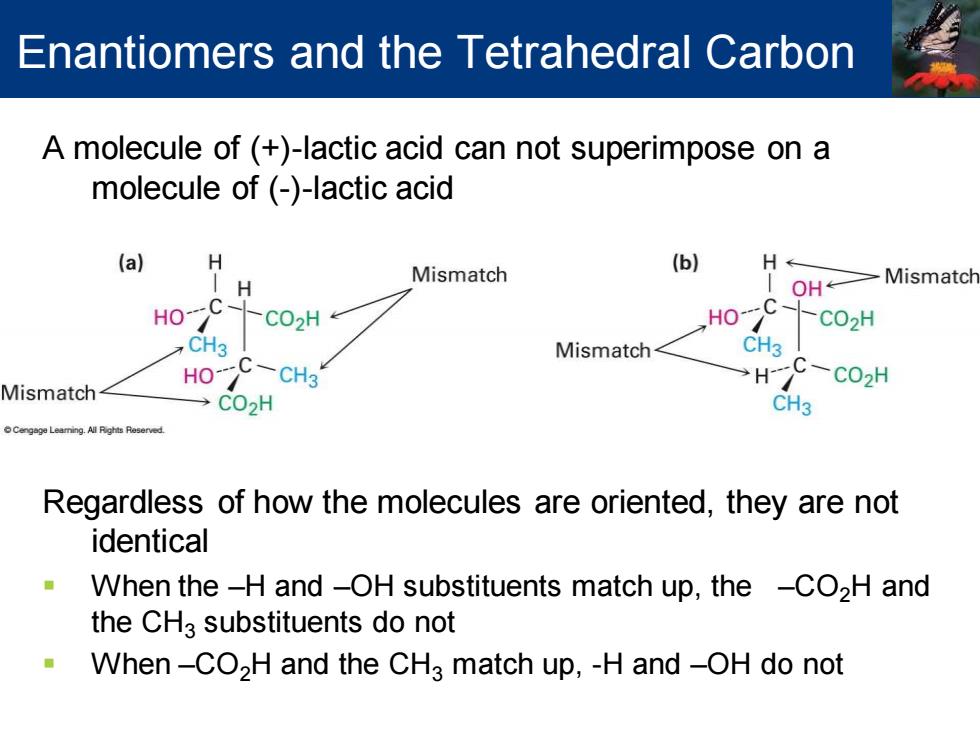
Enantiomers and the Tetrahedral Carbon A molecule of (+)-lactic acid can not superimpose on a molecule of (-)-lactic acid (a) Mismatch (b) H H OH Mismatch HO >C02H HO C -CO2H CH3 Mismatch C Mismatch HO-C-CH3 HC-CO2H →C02H CH3 Regardless of how the molecules are oriented,they are not identical When the-H and -OH substituents match up,the -CO2H and the CHa substituents do not When-CO2H and the CH3 match up,-H and-OH do not
A molecule of (+)-lactic acid can not superimpose on a molecule of (-)-lactic acid Regardless of how the molecules are oriented, they are not identical ▪ When the –H and –OH substituents match up, the –CO2H and the CH3 substituents do not ▪ When –CO2H and the CH3 match up, -H and –OH do not Enantiomers and the Tetrahedral Carbon
5-2 The Reason for Handedness in Molecules: Chirality Chiral -From the Greek cheir meaning "hand" Molecules that are not identical to their mirror images,and thus exist in two enantiomeric forms A molecule is not chiral if it has a plane of symmetry Plane of symmetry A plane that cuts through the middle of an object (or molecule)so that one half of the object is a mirror image of the other half
Chiral ▪ From the Greek cheir meaning “hand” ▪ Molecules that are not identical to their mirror images, and thus exist in two enantiomeric forms ▪ A molecule is not chiral if it has a plane of symmetry Plane of symmetry ▪ A plane that cuts through the middle of an object (or molecule) so that one half of the object is a mirror image of the other half 5-2 The Reason for Handedness in Molecules: Chirality
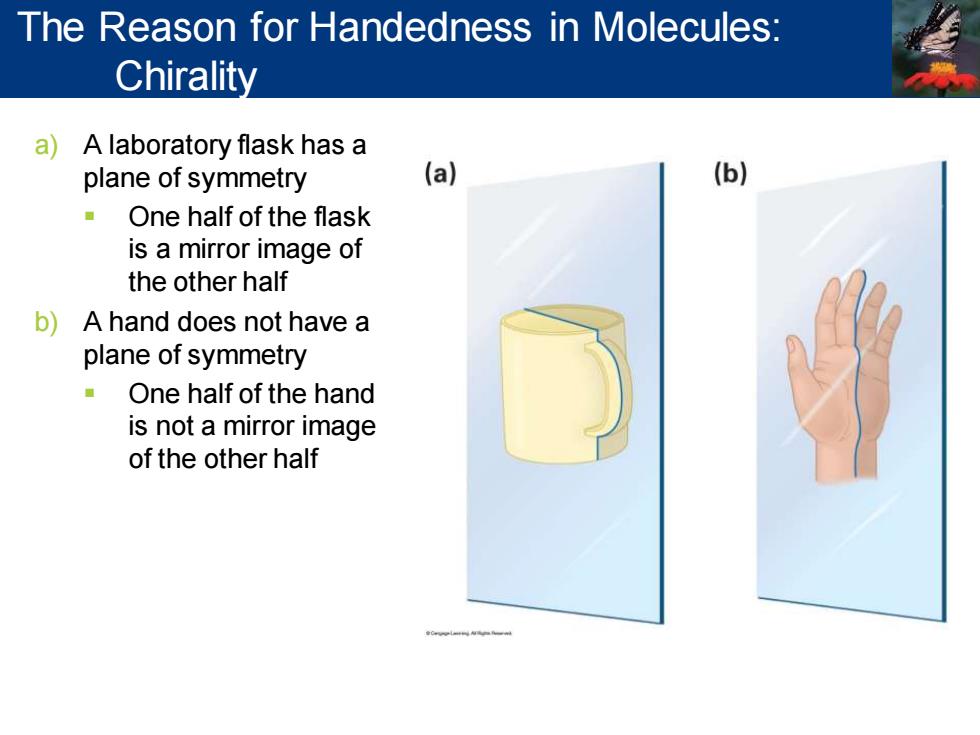
The Reason for Handedness in Molecules: Chirality a)A laboratory flask has a plane of symmetry (a) (b) One half of the flask is a mirror image of the other half b)A hand does not have a plane of symmetry One half of the hand is not a mirror image of the other half FORWLEINNAPm
a) A laboratory flask has a plane of symmetry ▪ One half of the flask is a mirror image of the other half b) A hand does not have a plane of symmetry ▪ One half of the hand is not a mirror image of the other half The Reason for Handedness in Molecules: Chirality
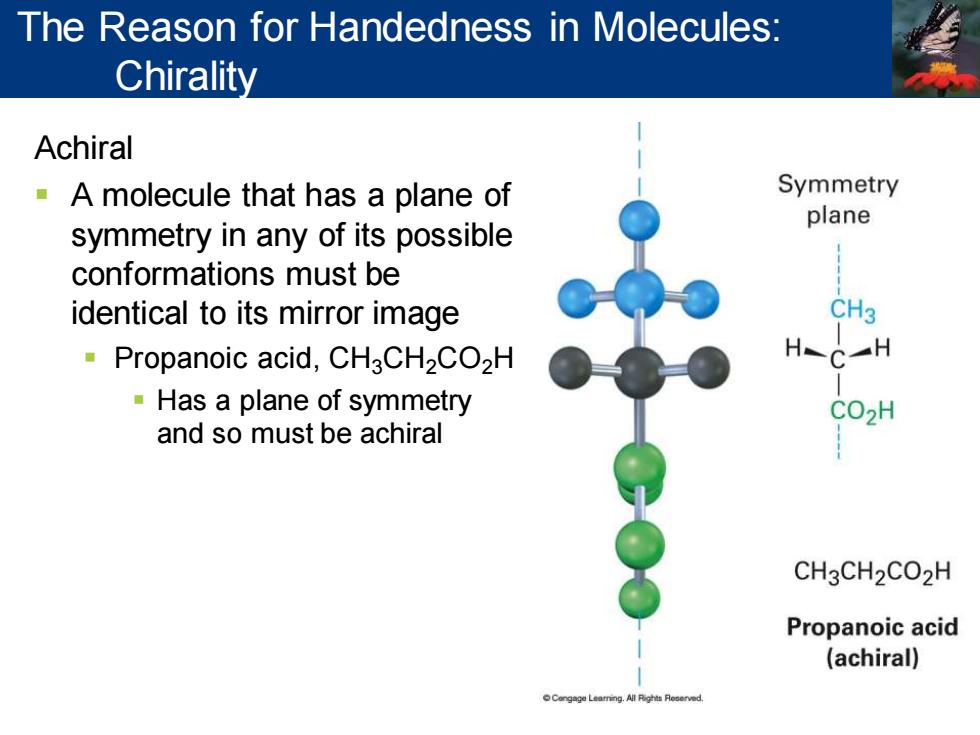
The Reason for Handedness in Molecules: Chirality Achiral -A molecule that has a plane of Symmetry plane symmetry in any of its possible conformations must be identical to its mirror image CH3 Propanoic acid,CH3CH2CO2H HC一H Has a plane of symmetry CO2H and so must be achiral CH3CH2CO2H Propanoic acid (achiral) Cengage Leaming.All Fighis Reoerved
Achiral ▪ A molecule that has a plane of symmetry in any of its possible conformations must be identical to its mirror image ▪ Propanoic acid, CH3CH2CO2H ▪ Has a plane of symmetry and so must be achiral The Reason for Handedness in Molecules: Chirality
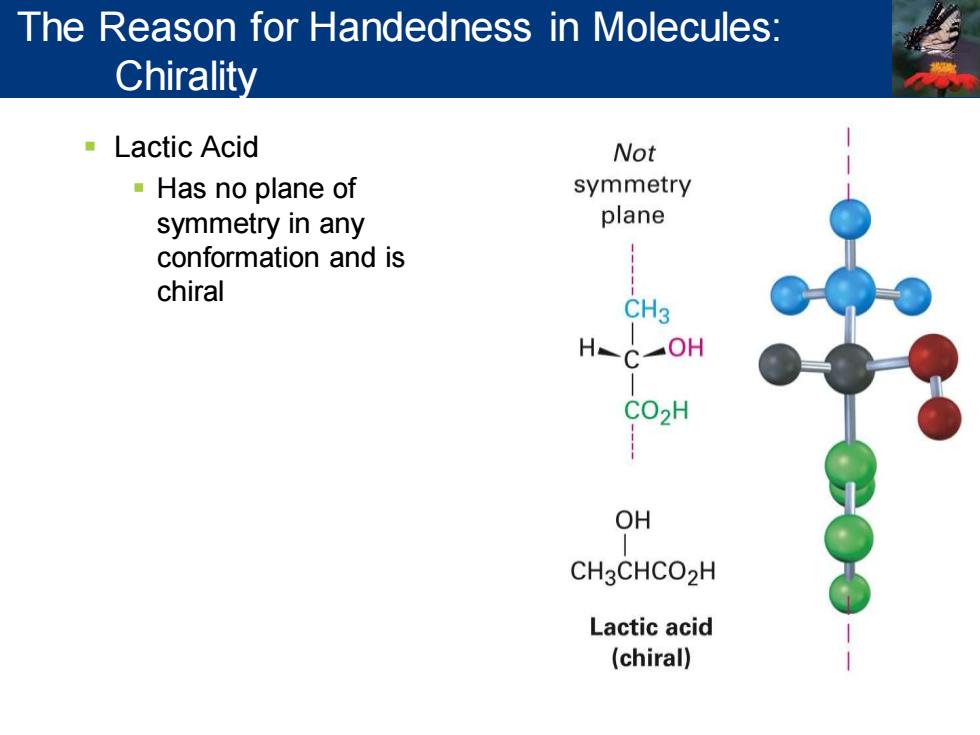
The Reason for Handedness in Molecules: Chirality ■Lactic Acid Not Has no plane of symmetry symmetry in any plane conformation and is chiral CH3 OH CO2H OH CH3CHCO2H Lactic acid (chiral)
▪ Lactic Acid ▪ Has no plane of symmetry in any conformation and is chiral The Reason for Handedness in Molecules: Chirality