
Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 6 Lecture Alkyl Halides: Nucleophilic Substitution and Elimination G.WADE,J R Rizalia Klausmeyer Baylor University Waco,TX 2013 Pearson Education,Inc ALWAYS LEARNING PEARSON
Chapter 6 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Alkyl Halides: Nucleophilic Substitution and Elimination © 2013 Pearson Education, Inc. Rizalia Klausmeyer Baylor University Waco, TX
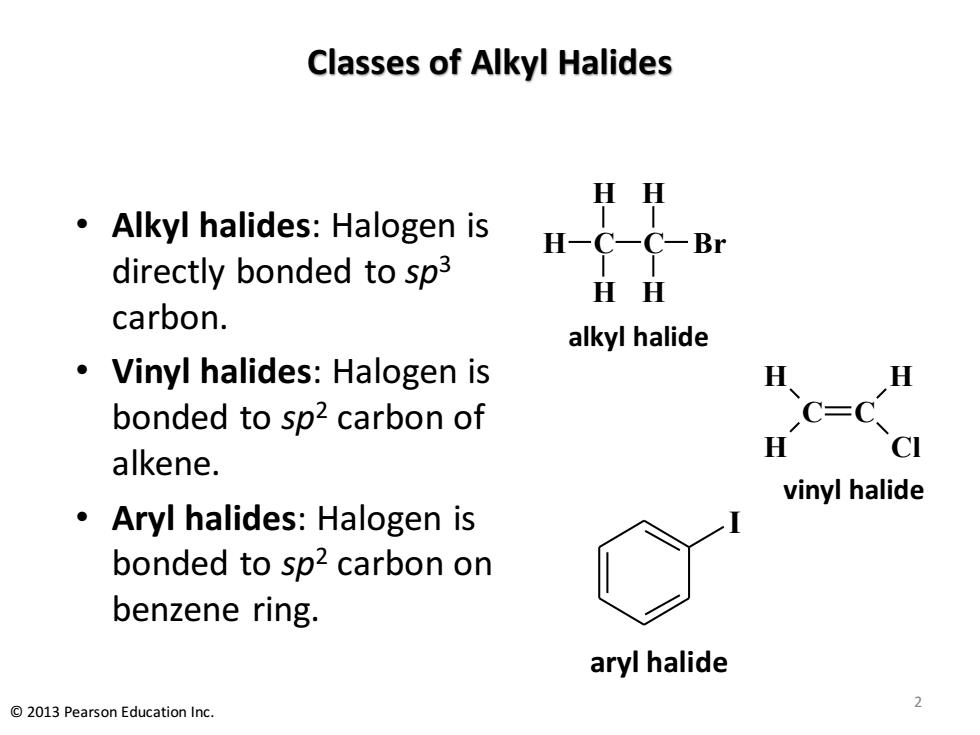
Classes of Alkyl Halides HH Alkyl halides:Halogen is H-C-C-Br directly bonded to sp3 HH carbon. alkyl halide Vinyl halides:Halogen is 及、 H bonded to sp2 carbon of C=C H CI alkene. vinyl halide Aryl halides:Halogen is bonded to sp2 carbon on benzene ring. aryl halide 2013 Pearson Education Inc
Classes of Alkyl Halides • Alkyl halides: Halogen is directly bonded to sp3 carbon. • Vinyl halides: Halogen is bonded to sp2 carbon of alkene. • Aryl halides: Halogen is bonded to sp2 carbon on benzene ring. C C H H H Cl vinyl halide C H H H C H H Br alkyl halide I aryl halide 2 © 2013 Pearson Education Inc
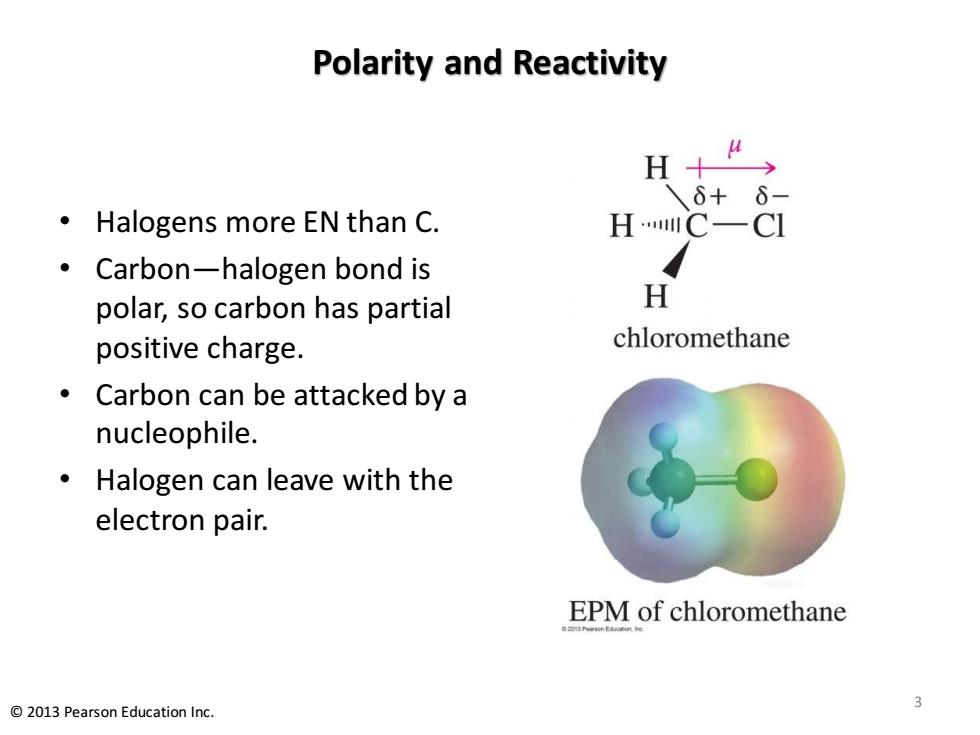
Polarity and Reactivity H+ 6+ 6 Halogens more EN than C. HC- CI Carbon-halogen bond is polar,so carbon has partial H positive charge. chloromethane Carbon can be attacked by a nucleophile. Halogen can leave with the electron pair. EPM of chloromethane 2013 Pearson Education Inc
Polarity and Reactivity • Halogens more EN than C. • Carbon—halogen bond is polar, so carbon has partial positive charge. • Carbon can be attacked by a nucleophile. • Halogen can leave with the electron pair. 3 © 2013 Pearson Education Inc
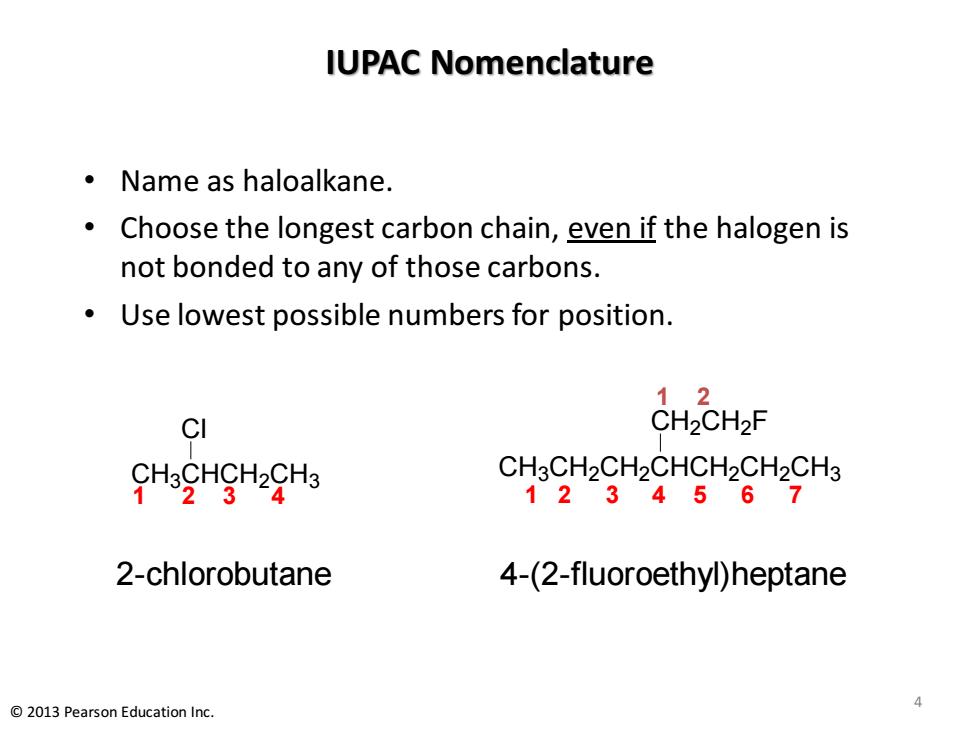
IUPAC Nomenclature ·Name as haloalkane. Choose the longest carbon chain,even if the halogen is not bonded to any of those carbons. Use lowest possible numbers for position. 12 CI CH2CH2F CHoCHgHgHa CH3CH2CH2CHCH2CH2CH3 1234567 2-chlorobutane 4-(2-fluoroethyl)heptane 2013 Pearson Education Inc
IUPAC Nomenclature • Name as haloalkane. • Choose the longest carbon chain, even if the halogen is not bonded to any of those carbons. • Use lowest possible numbers for position. CH3CH2CH2CHCH2CH2CH3 CH2CH2 F 1 2 3 4 2-chlorobutane 4-(2-fluoroethyl)heptane 1 2 3 4 5 6 7 1 2 CH3CHCH2CH3 Cl 4 © 2013 Pearson Education Inc
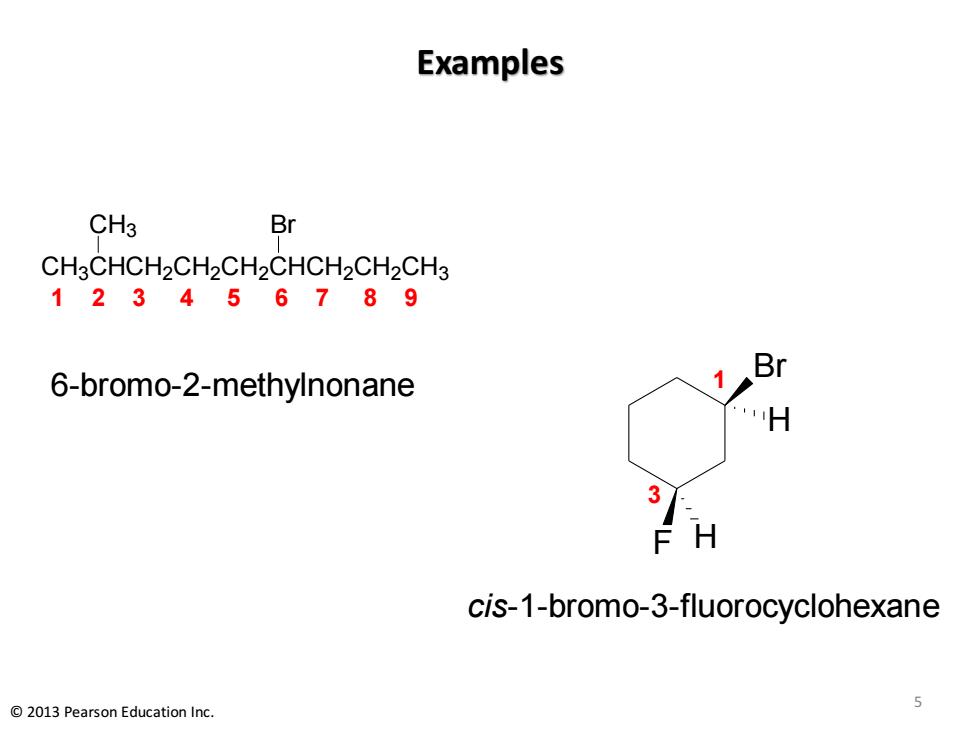
Examples CH3 Br CH3CHCH2CH2CH2CHCH2CH2CH3 123456789 Br 6-bromo-2-methylnonane H 3 FH cis-1-bromo-3-fluorocyclohexane 2013 Pearson Education Inc
Examples CH3CHCH2CH2CH2CHCH2CH2CH3 CH3 Br Br F H H 1 2 3 4 5 6 7 8 9 6-bromo-2-methylnonane 1 3 cis-1-bromo-3-fluorocyclohexane 5 © 2013 Pearson Education Inc