
CNGNGE JOHN MCMURRY CHAPTER 7 Alkenes and Alkynes EDITION Organic Chemistry with Biological Applications
CHAPTER 7 Alkenes and Alkynes
Alkenes and Alkynes Alkene (or olefin) Hydrocarbon that contains a carbon-carbon double bond Present in most organic and biological molecules Alkyne Hydrocarbon that contains a carbon-carbon triple bond Rarely occur in biological molecules or pathways
Alkenes and Alkynes Alkene (or olefin) ▪ Hydrocarbon that contains a carbon-carbon double bond ▪ Present in most organic and biological molecules Alkyne ▪ Hydrocarbon that contains a carbon-carbon triple bond ▪ Rarely occur in biological molecules or pathways
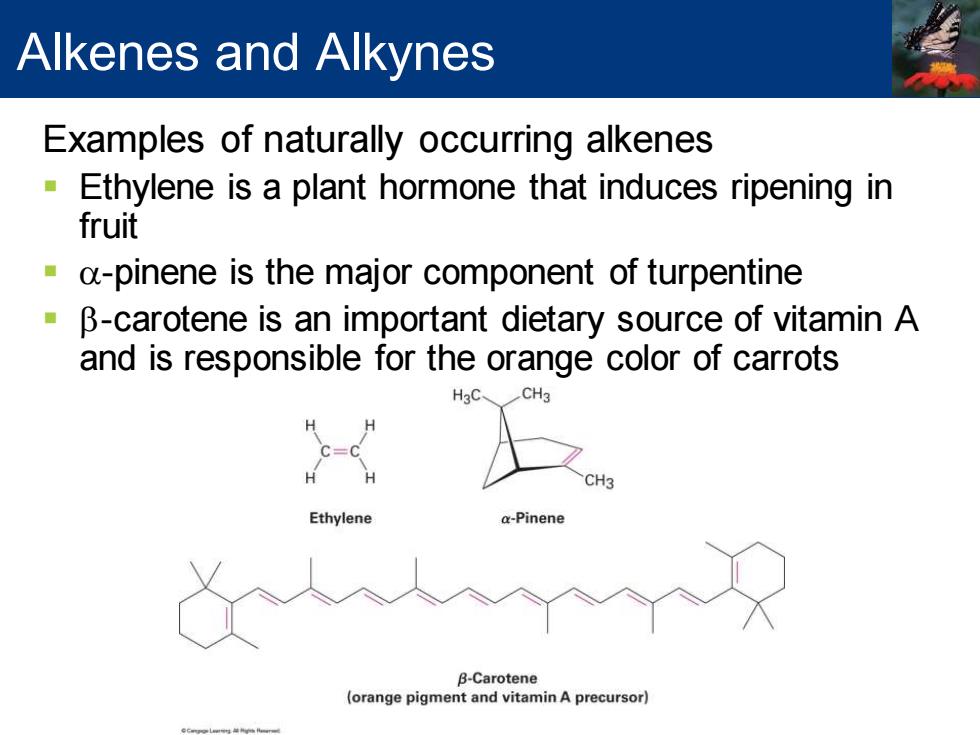
Alkenes and Alkynes Examples of naturally occurring alkenes Ethylene is a plant hormone that induces ripening in fruit a-pinene is the major component of turpentine B-carotene is an important dietary source of vitamin A and is responsible for the orange color of carrots CH3 Ethylene a-Pinene B-Carotene (orange pigment and vitamin A precursor)
Alkenes and Alkynes Examples of naturally occurring alkenes ▪ Ethylene is a plant hormone that induces ripening in fruit ▪ a-pinene is the major component of turpentine ▪ b-carotene is an important dietary source of vitamin A and is responsible for the orange color of carrots
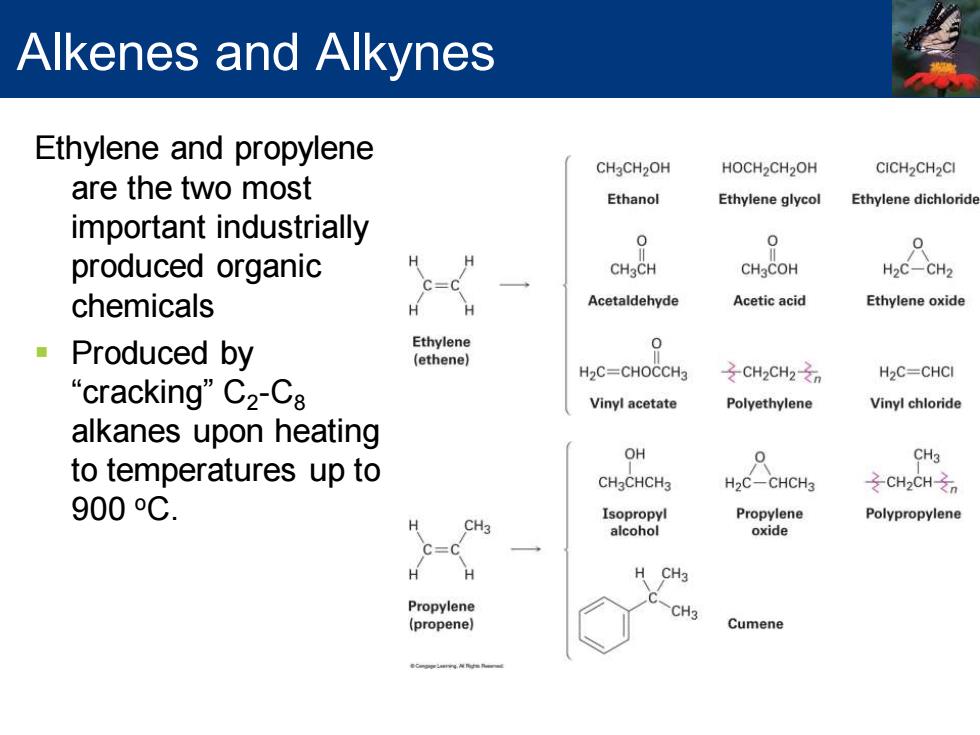
Alkenes and Alkynes Ethylene and propylene CH2CH2OH HOCH2CH2OH CICH2CH2CI are the two most Ethanol Ethylene glycol Ethylene dichloride important industrially 0 0 produced organic CHaCH CH3COH H2C-CH2 c=c chemicals H Acetaldehyde Acetic acid Ethylene oxide Produced by Ethylene 0 (ethene】 H2C=CHOCCH3 之CH2CH2之n H2C=CHCI “cracking”C2-C8 Vinyl acetate Polyethylene Vinyl chloride alkanes upon heating OH 0 CH3 to temperatures up to CH3CHCH3 H2C-CHCH3 之CH2CHzn 900oC. Isopropyl Propylene Polypropylene CH3 alcohol oxide H CH3 Propylene CH3 (propene) Cumene
Alkenes and Alkynes Ethylene and propylene are the two most important industrially produced organic chemicals ▪ Produced by “cracking” C2 -C8 alkanes upon heating to temperatures up to 900 oC
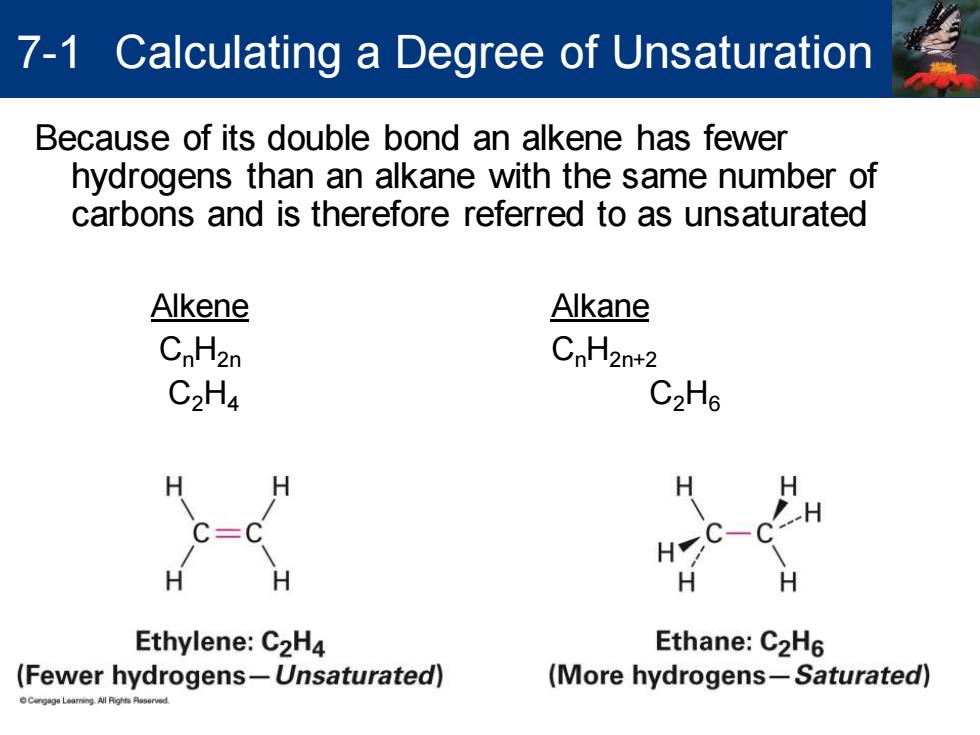
7-1 Calculating a Degree of Unsaturation Because of its double bond an alkene has fewer hydrogens than an alkane with the same number of carbons and is therefore referred to as unsaturated Alkene Alkane CnH2n CnH2n+2 C2H4 C2H6 H Ethylene:C2H4 Ethane:C2H6 (Fewer hydrogens-Unsaturated) (More hydrogens-Saturated)
Because of its double bond an alkene has fewer hydrogens than an alkane with the same number of carbons and is therefore referred to as unsaturated Alkene Alkane CnH2n CnH2n+2 C2H4 C2H6 7-1 Calculating a Degree of Unsaturation