
CNGNGE JOHN MCMURRY CHAPTER 5 Stereochemistry at Tetrahedral Centers T H I R D E DI TION Organic Chemistry with Biological Applications
CHAPTER 5 Stereochemistry at Tetrahedral Centers

Handedness Right and left hands are not identical Right and left hands are mirror images of each other they are nonsuperimposable mirror images Almost all the molecules in the human body are handed Handedness primarily arises from the tetrahedral stereochemistry of sp3-hybridized carbon atoms Handedness is crucial to understanding biological chemistry Left hand Right hand
Right and left hands are not identical ▪ Right and left hands are mirror images of each other – they are nonsuperimposable mirror images ▪ Almost all the molecules in the human body are handed ▪ Handedness primarily arises from the tetrahedral stereochemistry of sp3 -hybridized carbon atoms ▪ Handedness is crucial to understanding biological chemistry Handedness
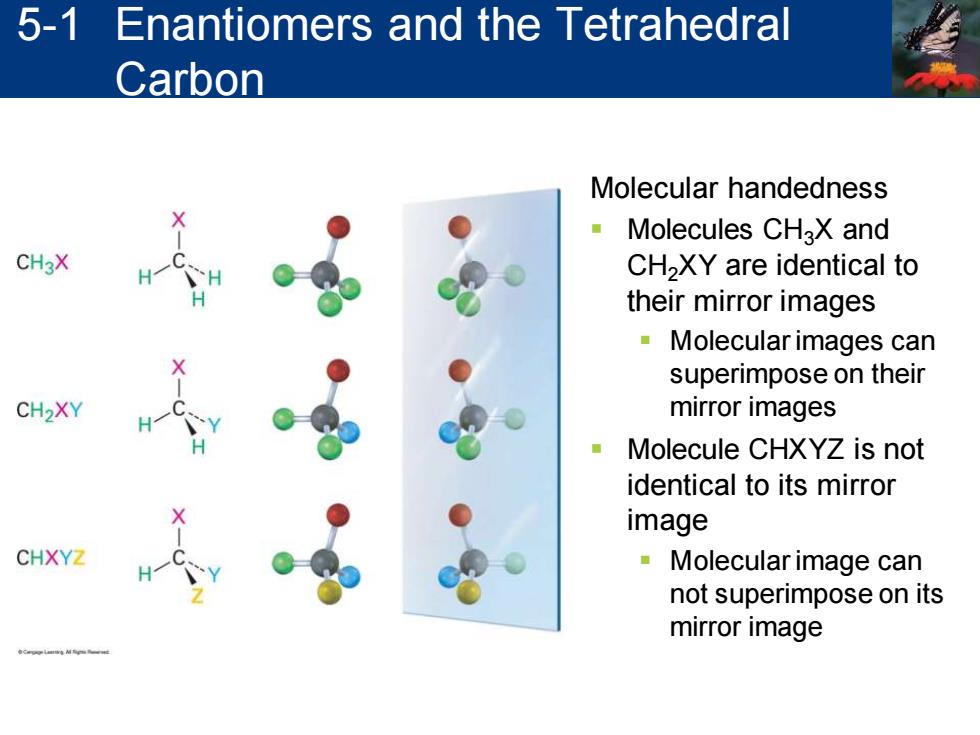
5-1 Enantiomers and the Tetrahedral Carbon Molecular handedness Molecules CHaX and CH3X CH2XY are identical to their mirror images 。Molecular images can superimpose on their CH2XY mirror images Molecule CHXYZ is not identical to its mirror image CHXYZ 。Molecular image can not superimpose on its mirror image
Molecular handedness ▪ Molecules CH3X and CH2XY are identical to their mirror images ▪ Molecular images can superimpose on their mirror images ▪ Molecule CHXYZ is not identical to its mirror image ▪ Molecular image can not superimpose on its mirror image 5-1 Enantiomers and the Tetrahedral Carbon
Enantiomers and the Tetrahedral Carbon Enantiomers "From the Greek enantio,meaning“opposite'” Stereoisomers in which molecules are not identical to their mirror images Result whenever a tetrahedral carbon is bonded to four different substituents CHXYZ (one need not be H) Lactic acid(2-hydroxypropanoic acid)has four different groups (-H,-OH,-CH3,-CO2H)bonded to the central carbon atoms and exists as a pair of enantiomers
Enantiomers ▪ From the Greek enantio, meaning “opposite” ▪ Stereoisomers in which molecules are not identical to their mirror images ▪ Result whenever a tetrahedral carbon is bonded to four different substituents CHXYZ (one need not be H) ▪ Lactic acid (2-hydroxypropanoic acid) has four different groups (-H, -OH, -CH3 , -CO2H) bonded to the central carbon atoms and exists as a pair of enantiomers Enantiomers and the Tetrahedral Carbon
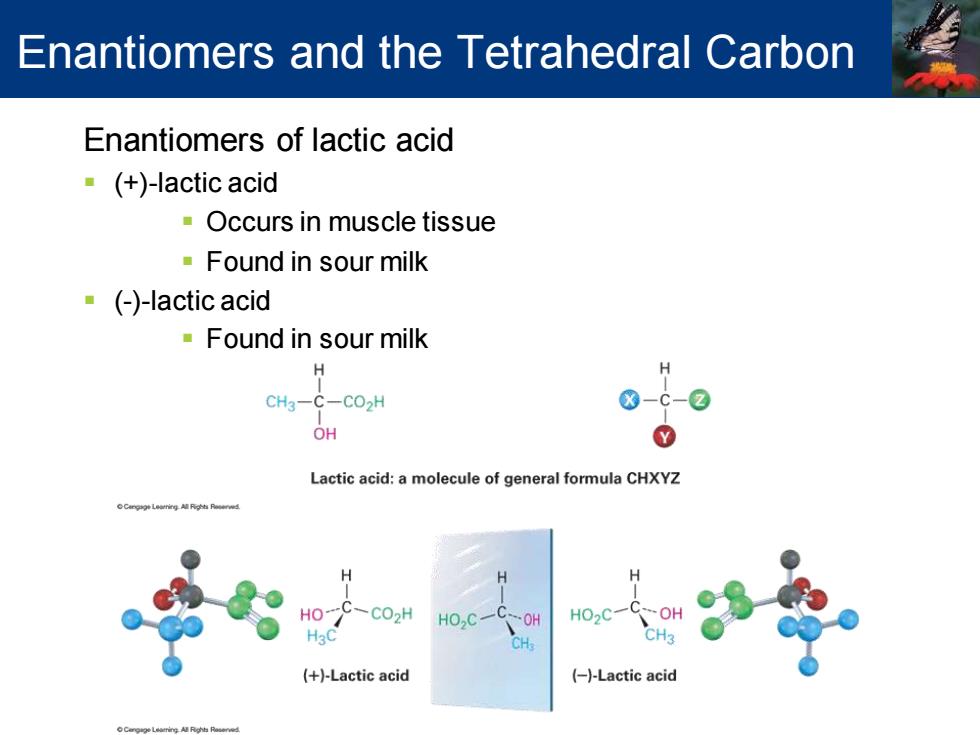
Enantiomers and the Tetrahedral Carbon Enantiomers of lactic acid (+)-lactic acid Occurs in muscle tissue Found in sour milk ()-lactic acid ·Found in sour milk H CH2- C-CO2H OH Lactic acid:a molecule of general formula CHXYZ H C02H HO,C- HO2C- (+)-Lactic acid (-)-Lactic acid
Enantiomers of lactic acid ▪ (+)-lactic acid ▪ Occurs in muscle tissue ▪ Found in sour milk ▪ (-)-lactic acid ▪ Found in sour milk Enantiomers and the Tetrahedral Carbon